

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM510049 ('WO2021205298, Compound 49 | (3S)-3-({N-[(4-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of His-tagged HIV-1 integrase-mediated 3' processing and strand transfer reactions using 5'-ACAGGCCTAGCACGCGTCG-Biotin-3' annealed with 5'... | Bioorg Med Chem Lett 25: 3013-6 (2015) Article DOI: 10.1016/j.bmcl.2015.05.011 BindingDB Entry DOI: 10.7270/Q2NG4TN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Magna Grecia di Catanzaro Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of wild type Human immunodeficiency virus 1 reverse transcriptase | Eur J Med Chem 50: 216-29 (2012) Article DOI: 10.1016/j.ejmech.2012.01.056 BindingDB Entry DOI: 10.7270/Q2G163Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM228619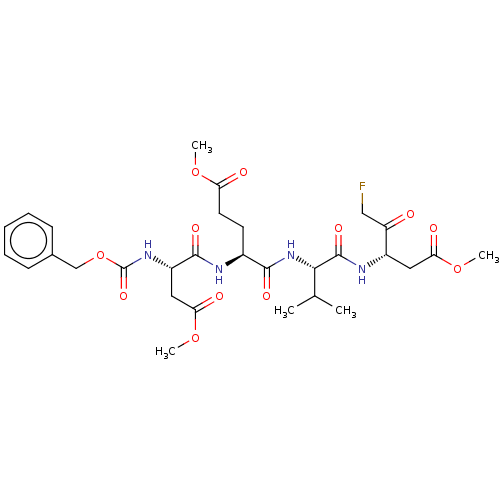 (US9345789, Z-DEVD-FMK) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM228619 (US9345789, Z-DEVD-FMK) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM46060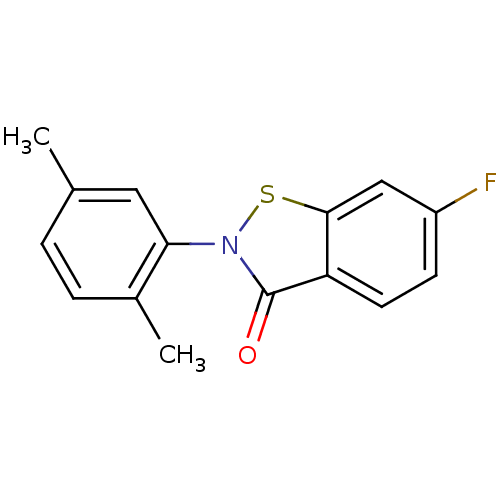 (2-(2,5-dimethylphenyl)-6-fluoranyl-1,2-benzothiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase-associated RNA-dependent DNA polymerase activity after 30 mins | Eur J Med Chem 93: 452-60 (2015) Article DOI: 10.1016/j.ejmech.2015.02.032 BindingDB Entry DOI: 10.7270/Q2BG2QPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485887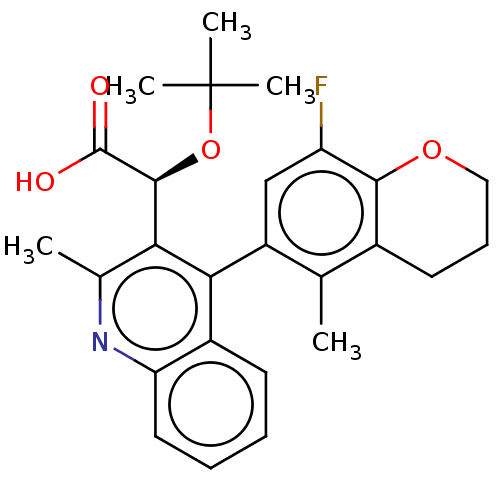 (CHEMBL2180481) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to recombinant HIV-1 integrase catalytic core domain expressed in Escherichia coli assessed as inhibition of interaction with LEDFG/... | Bioorg Med Chem Lett 25: 3013-6 (2015) Article DOI: 10.1016/j.bmcl.2015.05.011 BindingDB Entry DOI: 10.7270/Q2NG4TN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50076169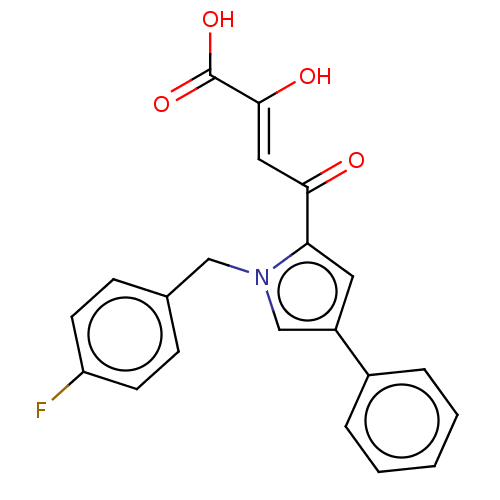 (CHEMBL3415853) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Siena | Assay Description RNA-dependent DNA polymerase (RDDP) activity was measured as described[J. Microbiol. 2015, 53:288-293] in Tris·HCl buffer (25 mL, 60 mm, pH 8.1) con... | Chembiochem 17: 683-8 (2016) Article DOI: 10.1002/cbic.201500668 BindingDB Entry DOI: 10.7270/Q25H7F28 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM46060 (2-(2,5-dimethylphenyl)-6-fluoranyl-1,2-benzothiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50076179 (CHEMBL3415843) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50076177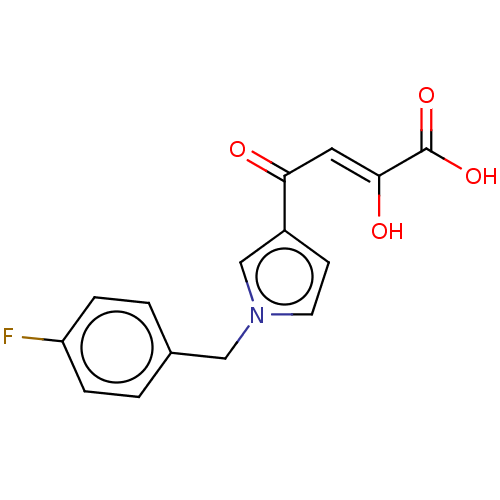 (CHEMBL3415845) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 8.1 | n/a |
University of Siena | Assay Description The RNA-dependent DNA polymerase (RDDP) activity associated with HIV-1 RT was measured by use of the Invitrogen EnzCheck Reverse Transcriptase Assay ... | Chembiochem 18: 374-377 (2017) Article DOI: 10.1002/cbic.201600592 BindingDB Entry DOI: 10.7270/Q2N29VS8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50142736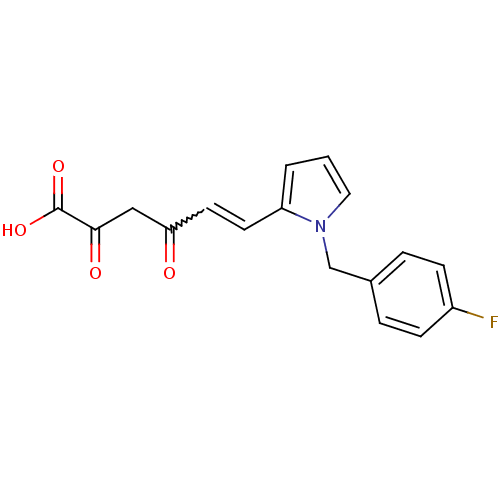 ((E)-6-[1-(4-Fluoro-benzyl)-1H-pyrrol-2-yl]-2,4-dio...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2337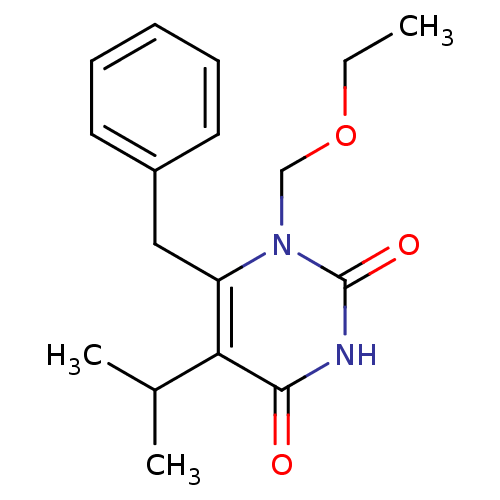 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442806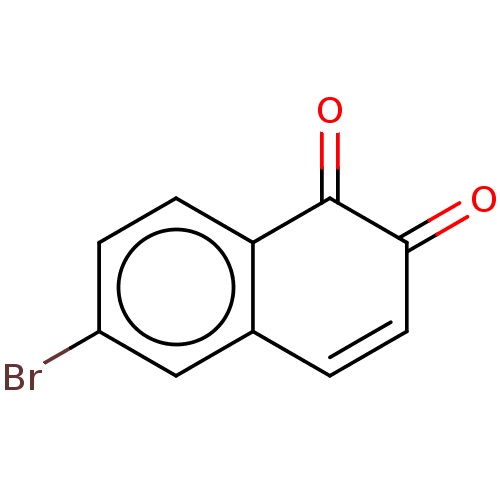 (Bonaphthone) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50494161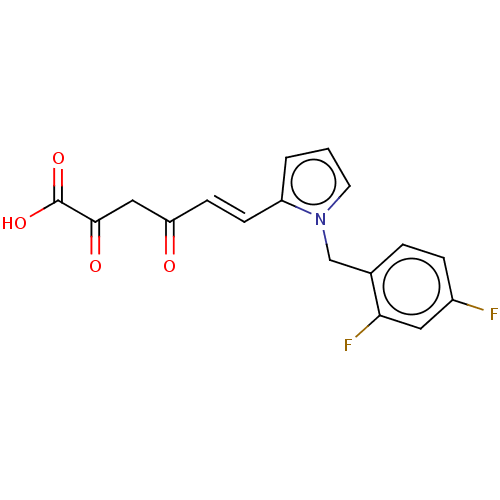 (CHEMBL3087617) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50076176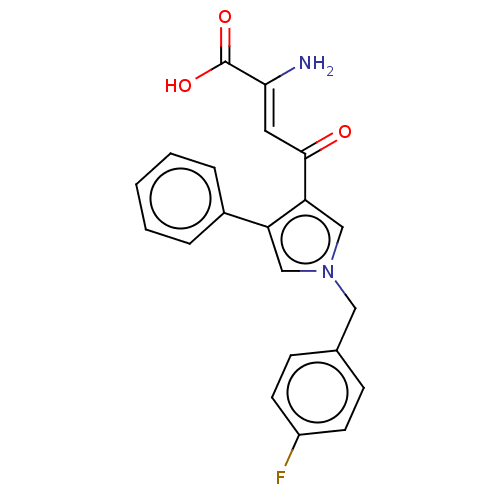 (CHEMBL3415846) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50596957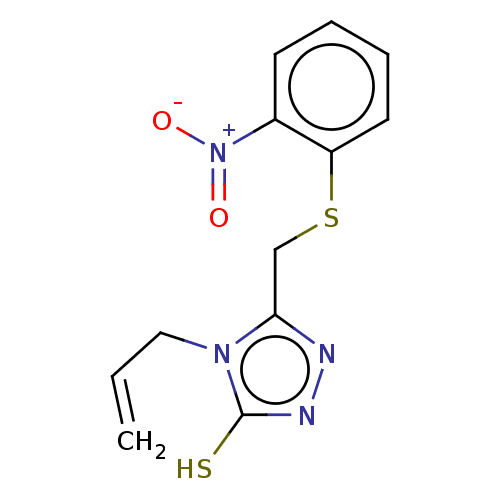 (CHEMBL1595621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00123 BindingDB Entry DOI: 10.7270/Q2TX3KCG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50587990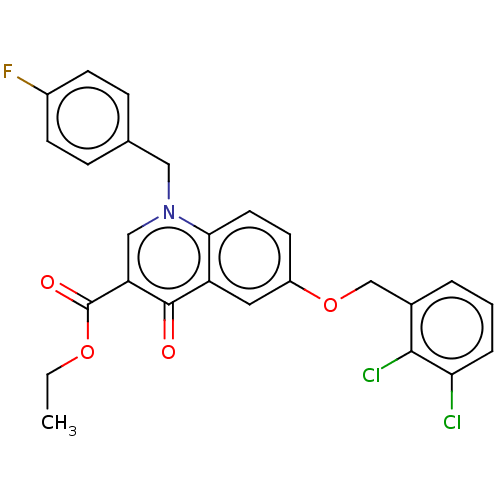 (CHEMBL5182942) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00535 BindingDB Entry DOI: 10.7270/Q2V98D1X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2335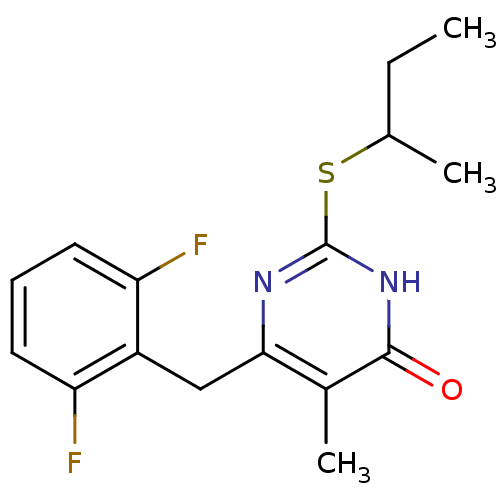 (2-(butan-2-ylsulfanyl)-6-[(2,6-difluorophenyl)meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2334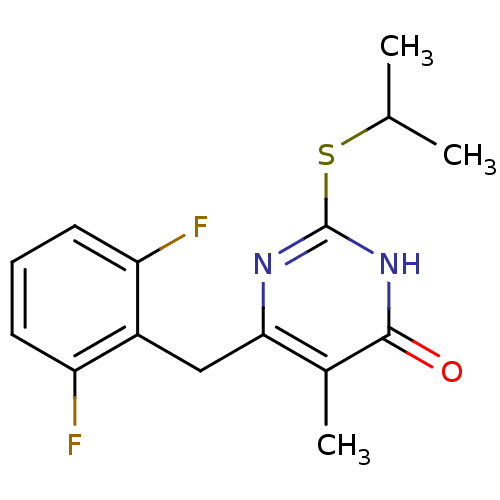 (6-[(2,6-difluorophenyl)methyl]-5-methyl-2-(propan-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2332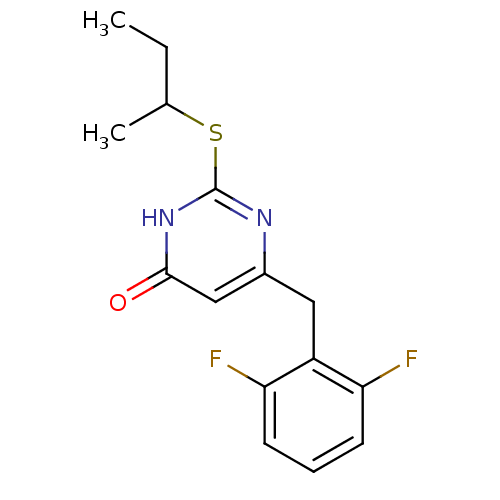 (2-(butan-2-ylsulfanyl)-6-[(2,6-difluorophenyl)meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2331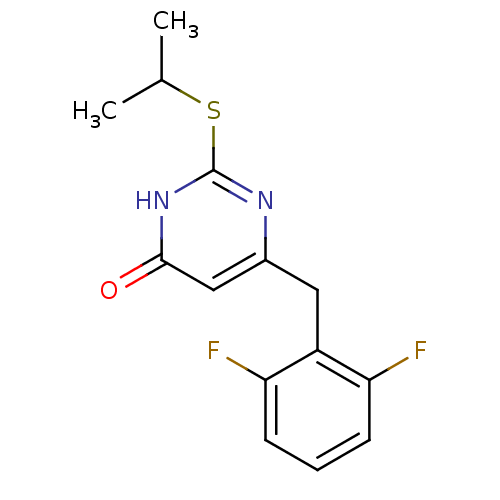 (6-[(2,6-difluorophenyl)methyl]-2-(propan-2-ylsulfa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50494183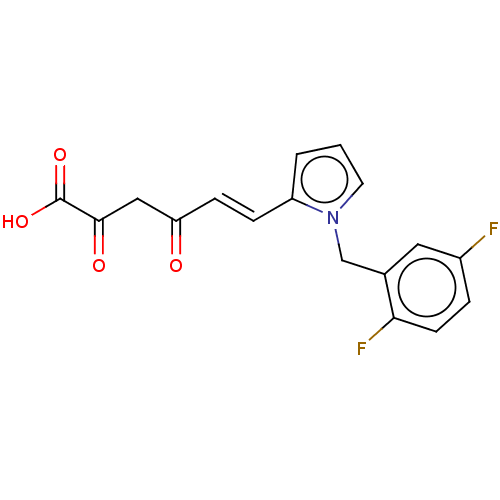 (CHEMBL3087618) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of His-tagged HIV-1 integrase-mediated 3' processing and strand transfer reactions using 5'-ACAGGCCTAGCACGCGTCG-Biotin-3' annealed with 5'... | Bioorg Med Chem Lett 25: 3013-6 (2015) Article DOI: 10.1016/j.bmcl.2015.05.011 BindingDB Entry DOI: 10.7270/Q2NG4TN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM23399 (4-{1-[(4-fluorophenyl)methyl]-1H-pyrrol-2-yl}-2,4-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of LEDGF/p75-dependent full length HIV-1 integrase expressed in Escherichia coli BL21(DE3) cells preincubated for 1 hr further incubated f... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111617 BindingDB Entry DOI: 10.7270/Q2NK3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50494162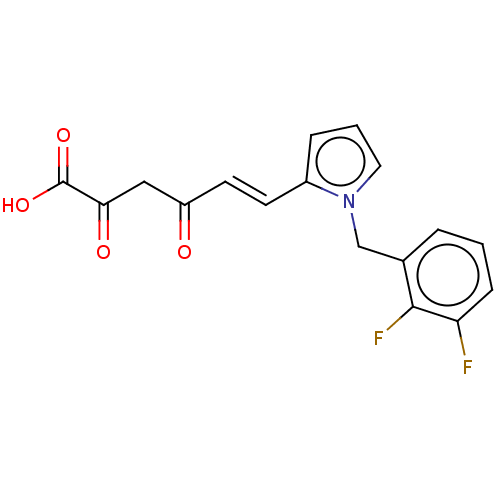 (CHEMBL3087616) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2383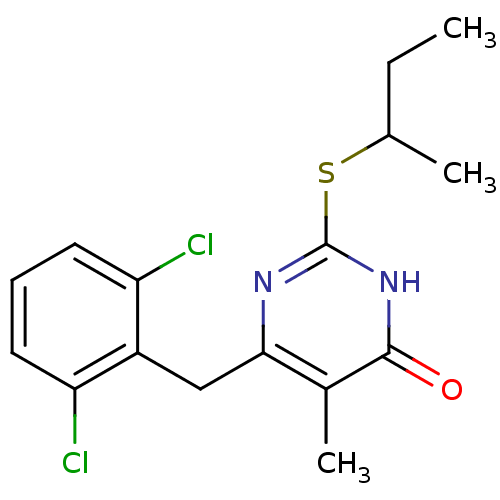 (2-(butan-2-ylsulfanyl)-6-[(2,6-dichlorophenyl)meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011006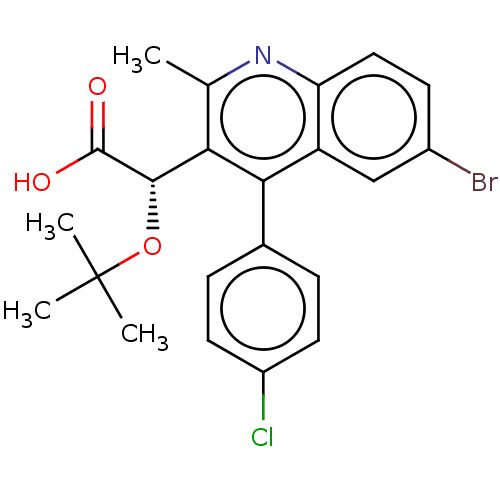 (CHEMBL3259895) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to recombinant HIV-1 integrase catalytic core domain expressed in Escherichia coli assessed as inhibition of interaction with LEDFG/... | Bioorg Med Chem Lett 25: 3013-6 (2015) Article DOI: 10.1016/j.bmcl.2015.05.011 BindingDB Entry DOI: 10.7270/Q2NG4TN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50076175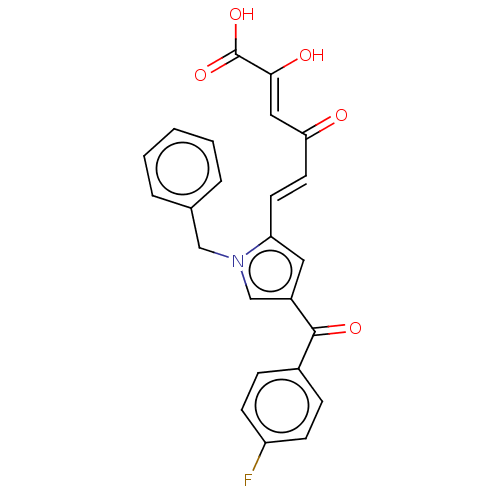 (CHEMBL3415847) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50076172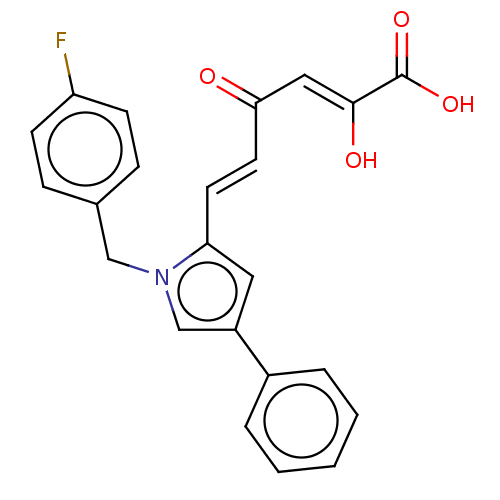 (CHEMBL3415850) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2392 (2-(cyclohexylsulfanyl)-6-[(2,6-difluorophenyl)meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM23399 (4-{1-[(4-fluorophenyl)methyl]-1H-pyrrol-2-yl}-2,4-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 HXB2 integrase using 32P-labelled oligonucleotide as substrate after 1 hr by phosphorimager analysis | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442805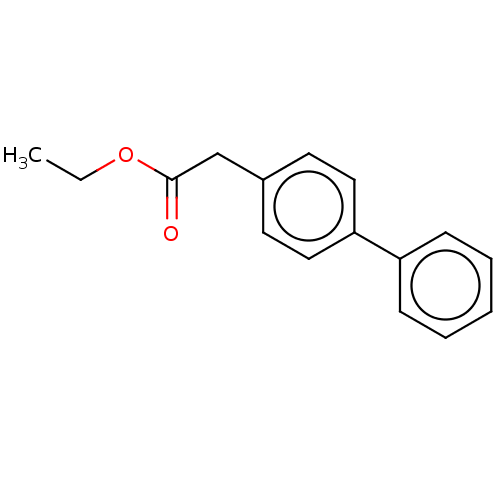 (Felbinac ethyl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50166940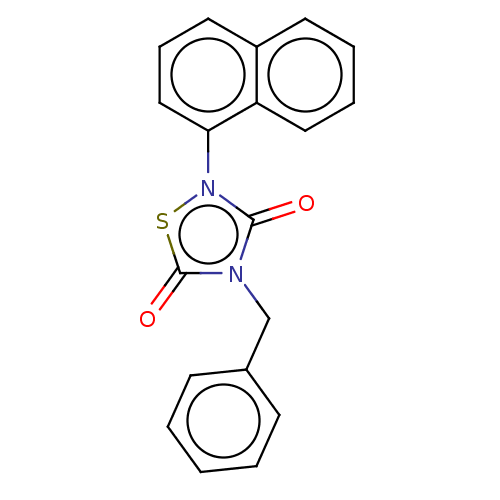 (NP-031112 | NP-12 | Tideglusib | US20230414581, Co...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2336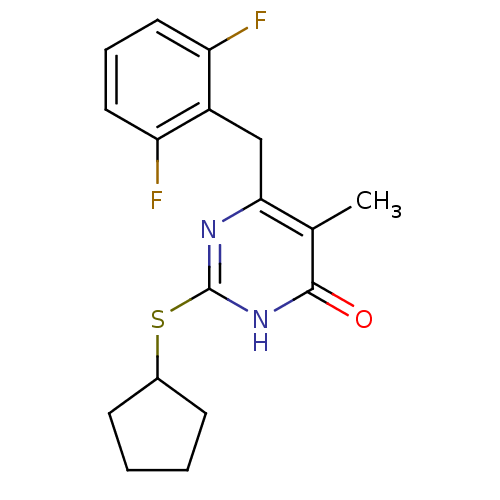 (2-(cyclopentylsulfanyl)-6-[(2,6-difluorophenyl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2333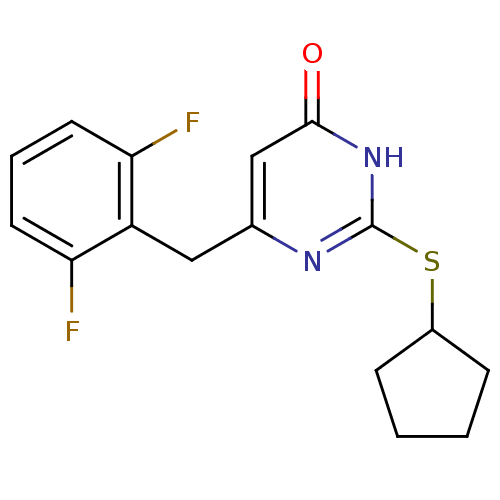 (2-(cyclopentylsulfanyl)-6-[(2,6-difluorophenyl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442806 (Bonaphthone) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD) | Assay Description Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... | bioRxiv 2020: (2020) Article DOI: 10.1101/2020.12.16.422677 BindingDB Entry DOI: 10.7270/Q26M39VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2389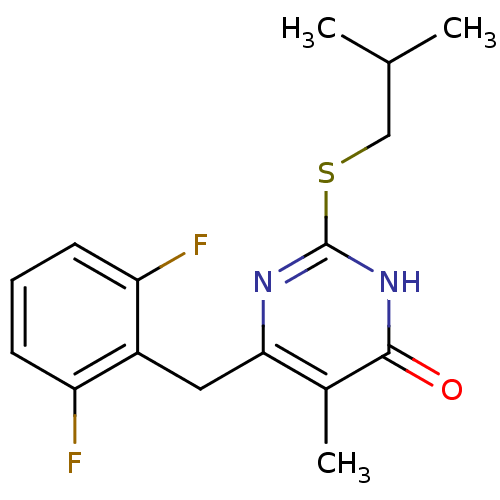 (6-[(2,6-difluorophenyl)methyl]-5-methyl-2-[(2-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 752 total ) | Next | Last >> |