

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM249581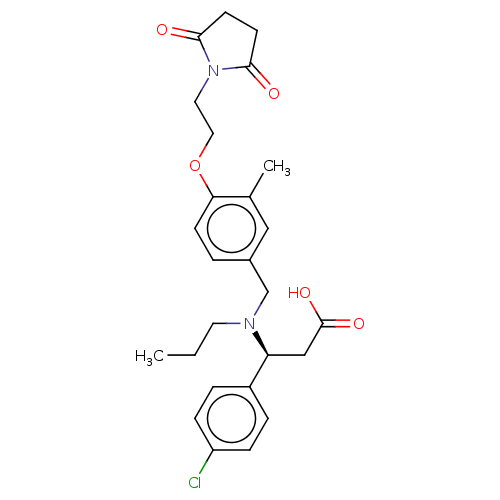 (US9447038, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249581 (US9447038, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249658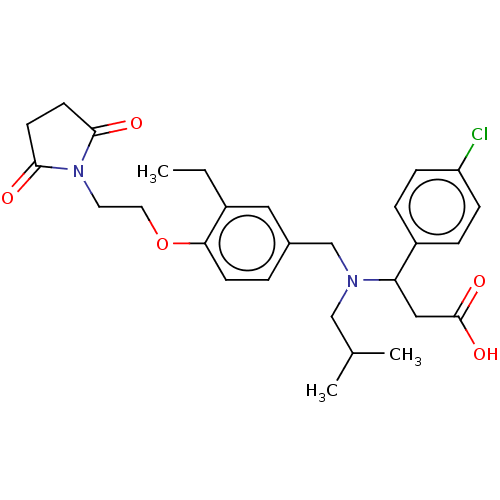 (US9447038, 86) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166453 (US9073853, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166453 (US9073853, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5418-5428 (2016) Article DOI: 10.1016/j.bmcl.2016.10.035 BindingDB Entry DOI: 10.7270/Q28S4RW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166545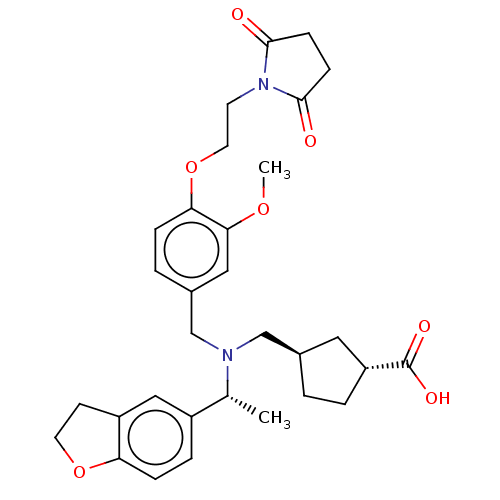 (US9073853, 104) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249583 (US9447038, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166446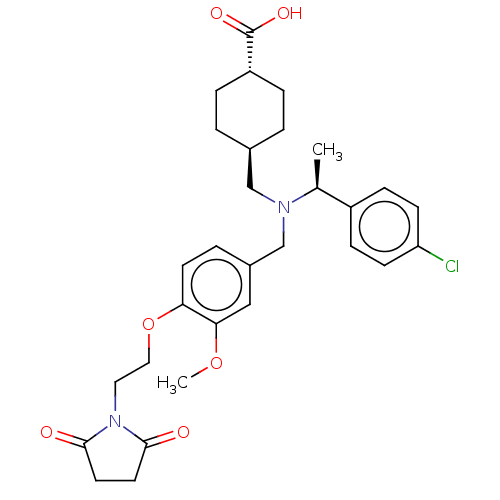 (US9073853, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5418-5428 (2016) Article DOI: 10.1016/j.bmcl.2016.10.035 BindingDB Entry DOI: 10.7270/Q28S4RW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166446 (US9073853, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166528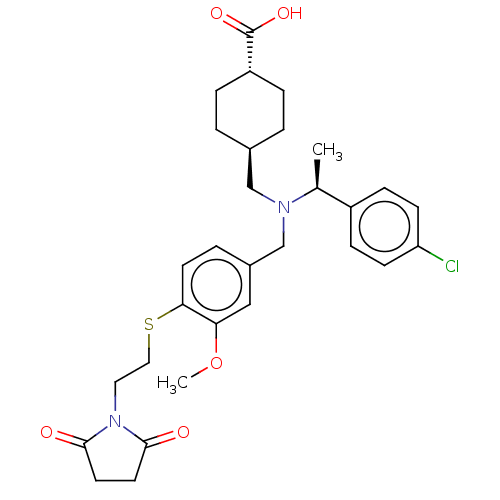 (US9073853, 87) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM249583 (US9447038, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM507637 (1-(2,3-dihydro-1,4-benzodioxin-6-yl)-5-[[4-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TQ64P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249763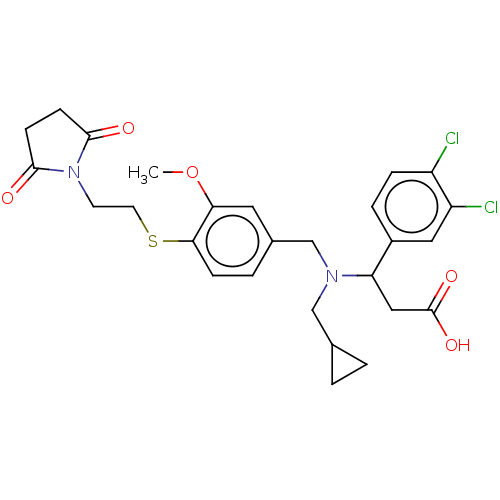 (US9447038, 191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249608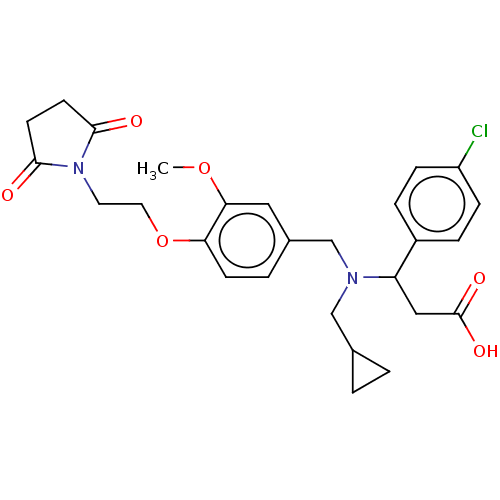 (US9447038, 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249609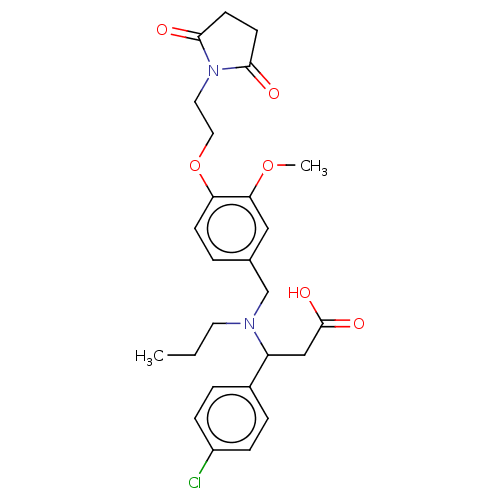 (US9447038, 37 | US9447038, 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166447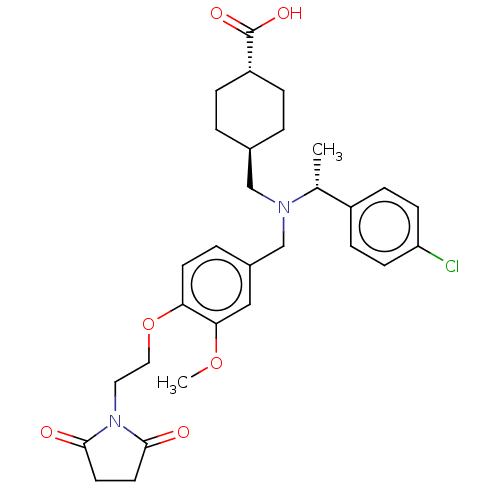 (US9073853, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50200716 (CHEMBL3916386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166475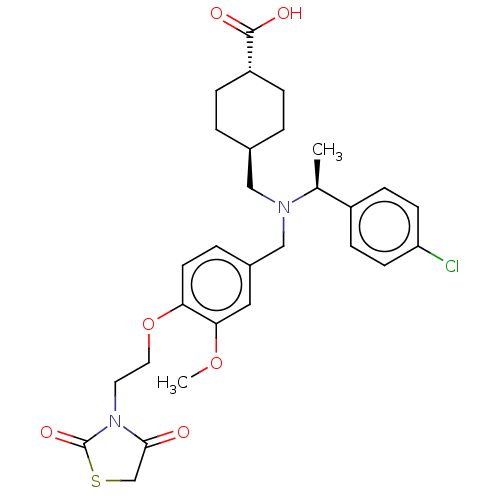 (US9073853, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM249577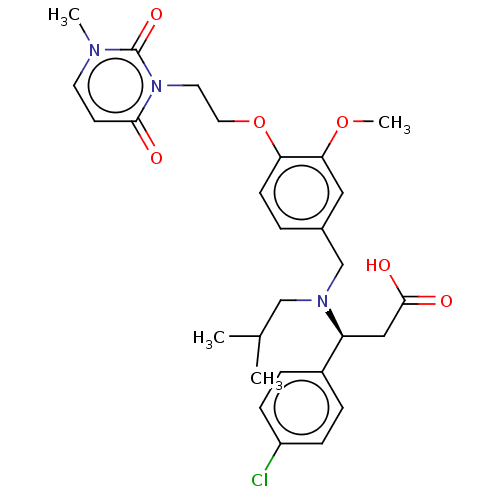 (US9447038, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166529 (US9073853, 88) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249577 (US9447038, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166529 (US9073853, 88) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5418-5428 (2016) Article DOI: 10.1016/j.bmcl.2016.10.035 BindingDB Entry DOI: 10.7270/Q28S4RW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249615 (US9447038, 43) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166451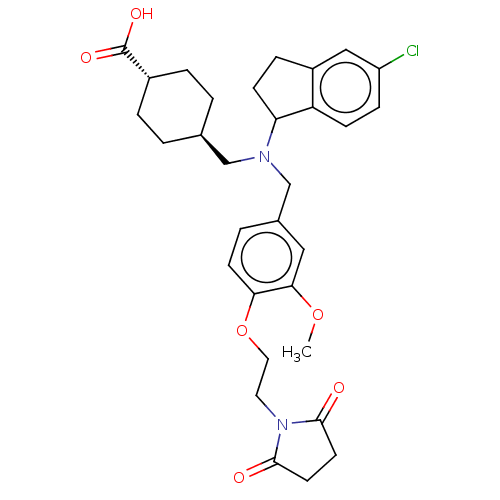 (US9073853, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5418-5428 (2016) Article DOI: 10.1016/j.bmcl.2016.10.035 BindingDB Entry DOI: 10.7270/Q28S4RW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166466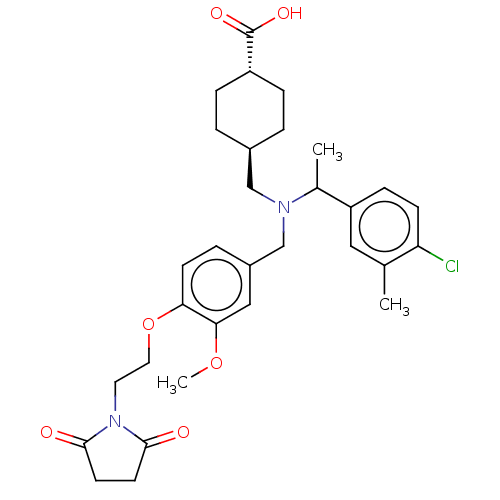 (US9073853, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166455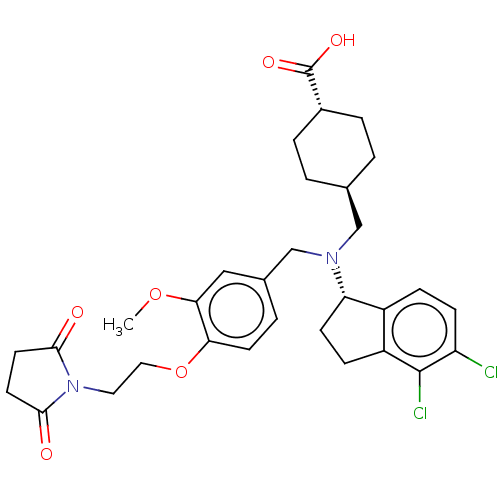 (US9073853, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166455 (US9073853, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5418-5428 (2016) Article DOI: 10.1016/j.bmcl.2016.10.035 BindingDB Entry DOI: 10.7270/Q28S4RW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249647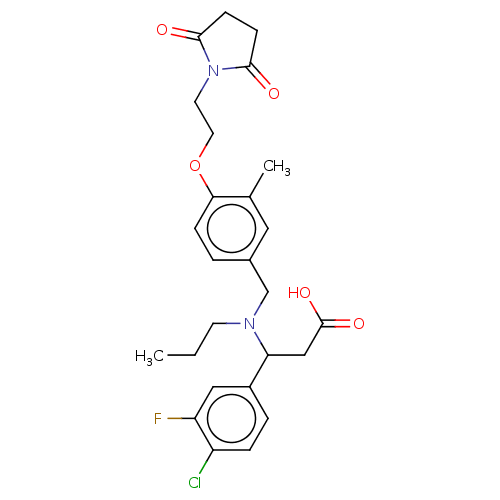 (US9447038, 75) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166589 (US9073853, 148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5418-5428 (2016) Article DOI: 10.1016/j.bmcl.2016.10.035 BindingDB Entry DOI: 10.7270/Q28S4RW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166589 (US9073853, 148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249720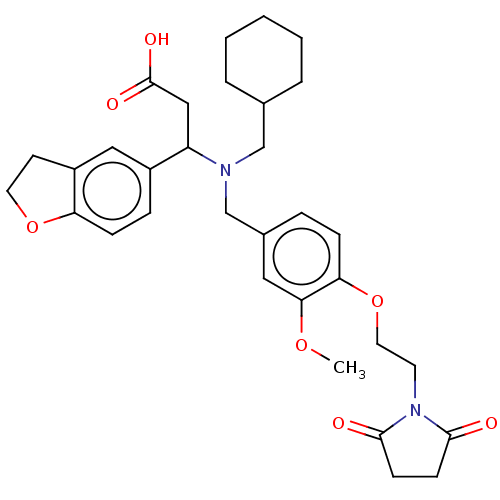 (US9447038, 148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166477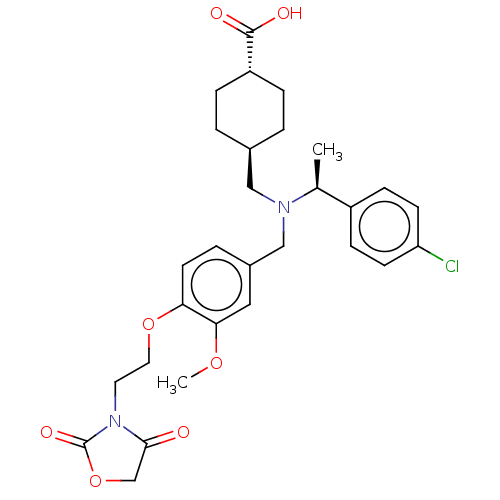 (US9073853, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM507626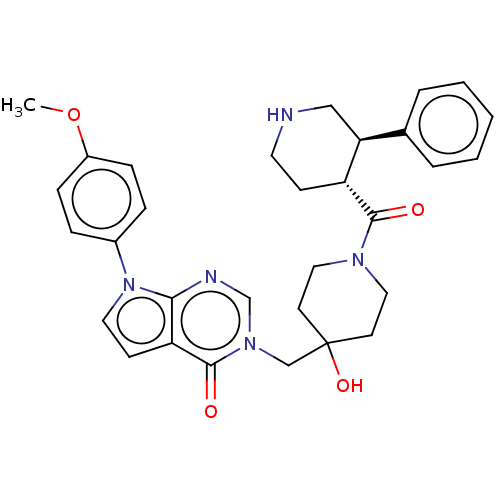 (3-[[4-hydroxy-1-[(3R,4R)-3-phenylpiperidine-4-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TQ64P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249696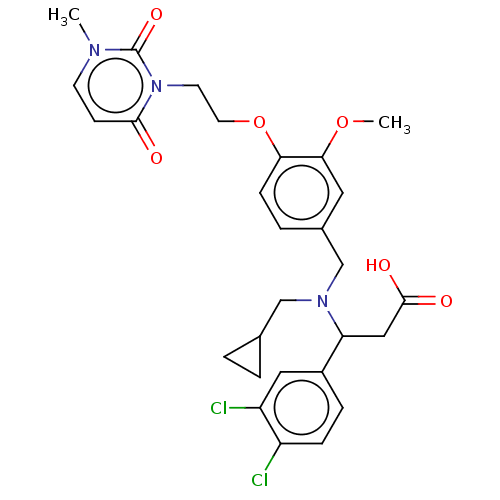 (US9447038, 124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM249600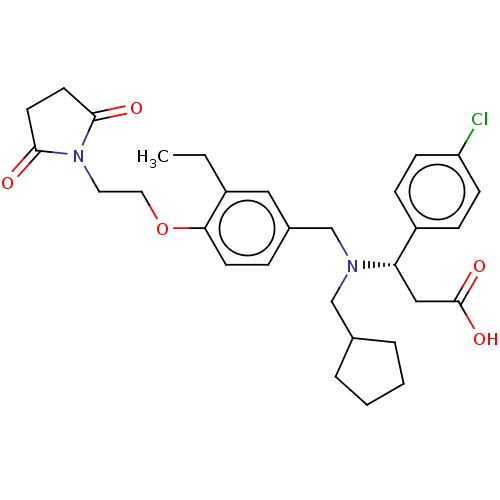 (US9447038, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249601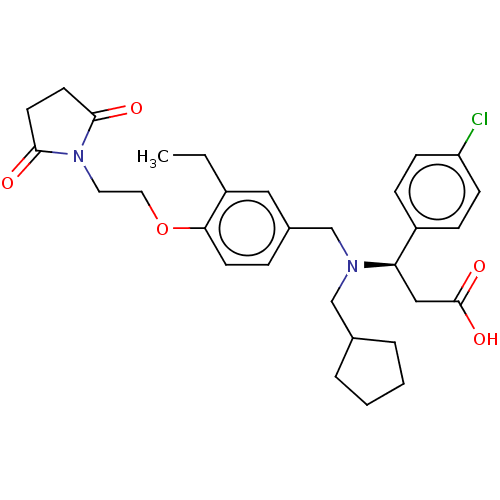 (US9447038, 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249760 (US9447038, 188) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249659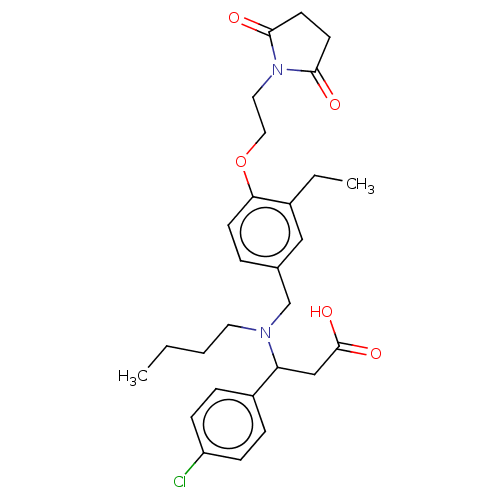 (US9447038, 87) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM507620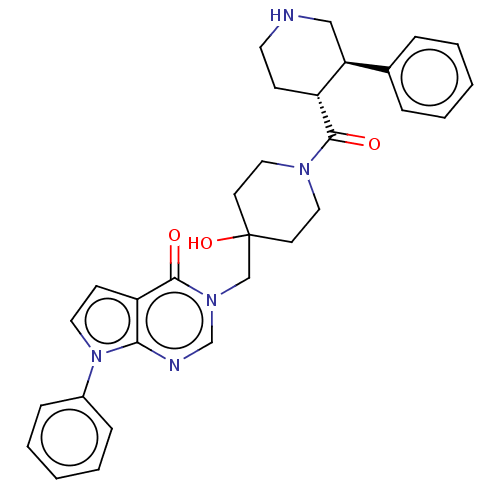 (3-[[4-hydroxy-1-[(3R,4R)-3-phenylpiperidine-4-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TQ64P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249596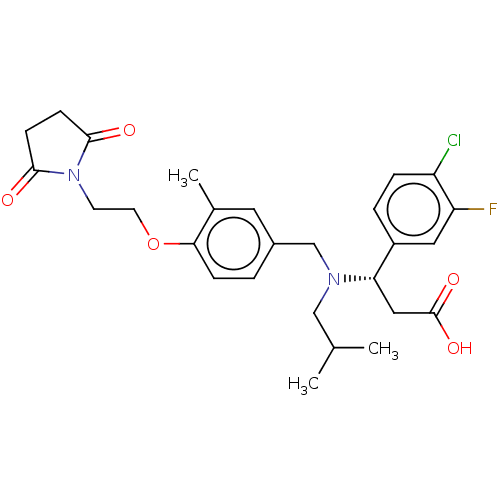 (US9447038, 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166450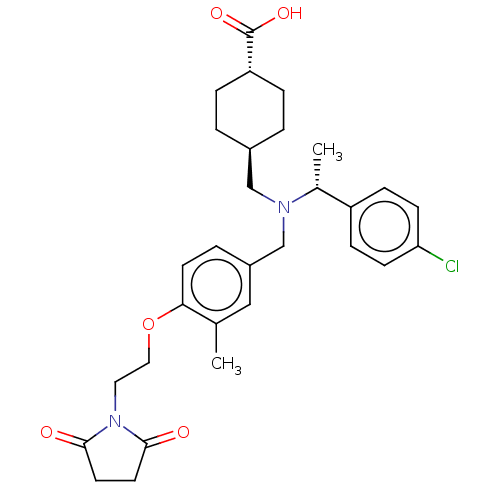 (US9073853, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM249596 (US9447038, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249646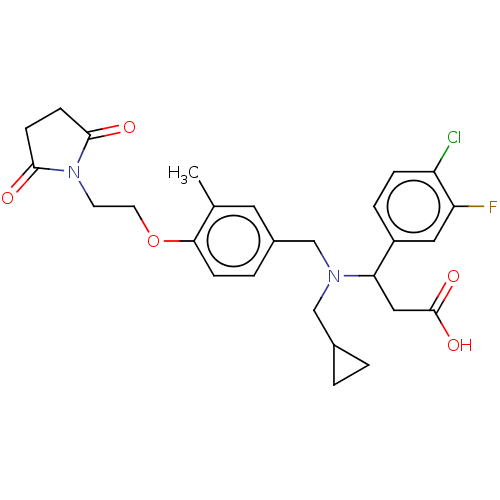 (US9447038, 74) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C motif chemokine 10 (Homo sapiens (Human)) | BDBM249574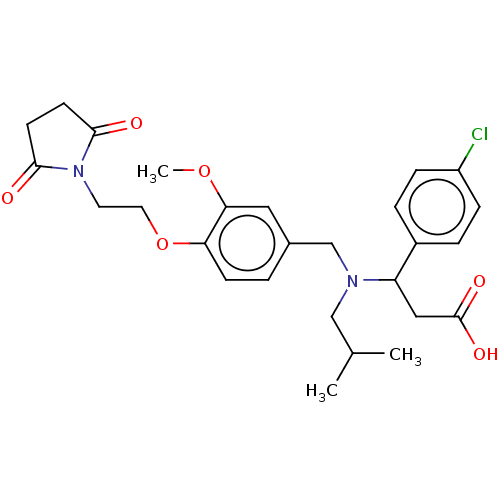 (US9447038, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description The composition of the binding assay buffer is determined in a course of detailed optimization procedure. This resulted in a binding assay buffer con... | US Patent US9447038 (2016) BindingDB Entry DOI: 10.7270/Q29G5KQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM249574 (US9447038, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166457 (US9073853, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5418-5428 (2016) Article DOI: 10.1016/j.bmcl.2016.10.035 BindingDB Entry DOI: 10.7270/Q28S4RW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166457 (US9073853, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50200713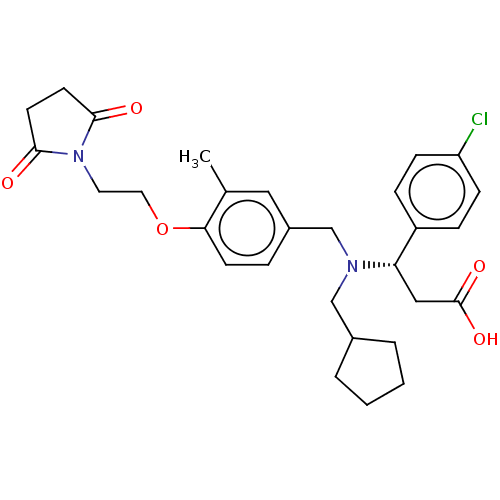 (CHEMBL3915994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL10 from human recombinant CXCR3 transfected in Flp-In-CHO cell membranes after 60 mins by gamma counting method | Bioorg Med Chem Lett 26: 5429-5437 (2016) Article DOI: 10.1016/j.bmcl.2016.10.038 BindingDB Entry DOI: 10.7270/Q2514164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166445 (US9073853, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM166456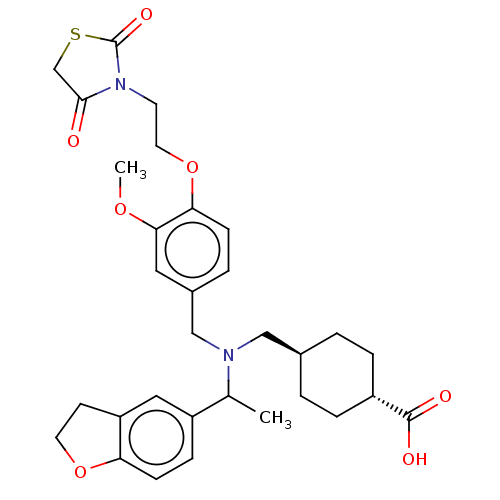 (US9073853, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Competition radioligand binding assays were performed to determine the in vitro potency of the newly synthesized, unlabeled test compounds to displac... | US Patent US9073853 (2015) BindingDB Entry DOI: 10.7270/Q2Q23Z10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 529 total ) | Next | Last >> |