

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036257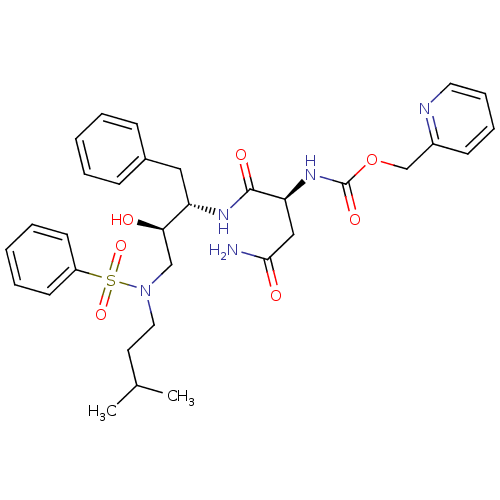 (((S)-1-{(1S,2R)-3-[Benzenesulfonyl-(3-methyl-butyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036261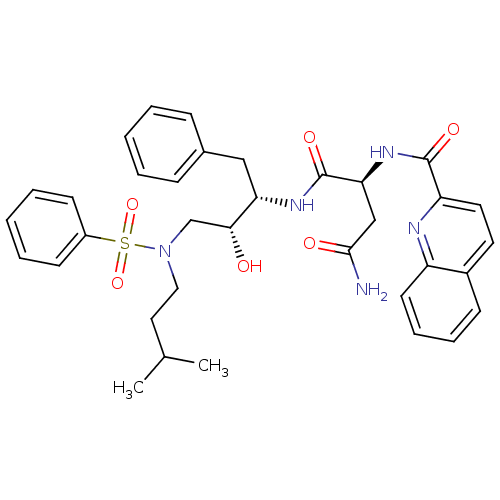 ((S)-N*1*-{(1S,2R)-3-[Benzenesulfonyl-(3-methyl-but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036251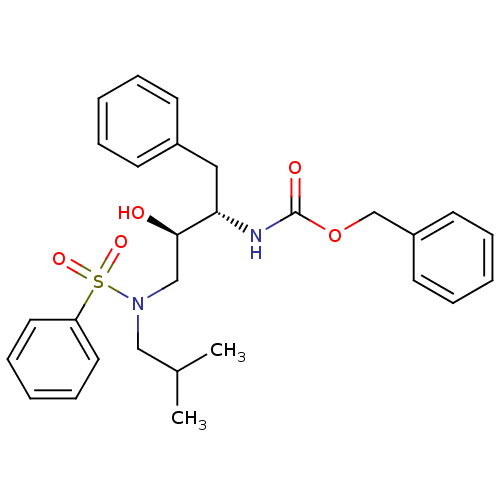 (CHEMBL141265 | [(1S,2R)-3-(Benzenesulfonyl-isobuty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036252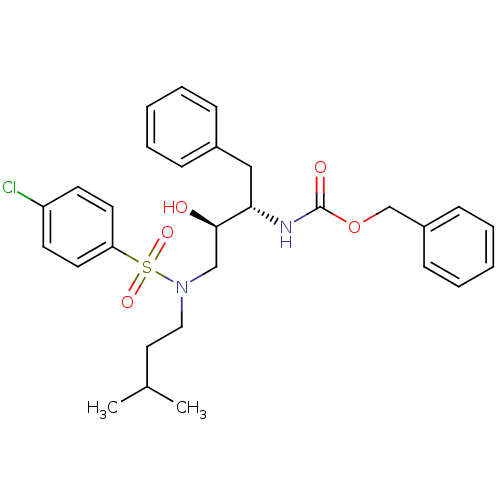 (CHEMBL347671 | {(1S,2R)-1-Benzyl-3-[(4-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036262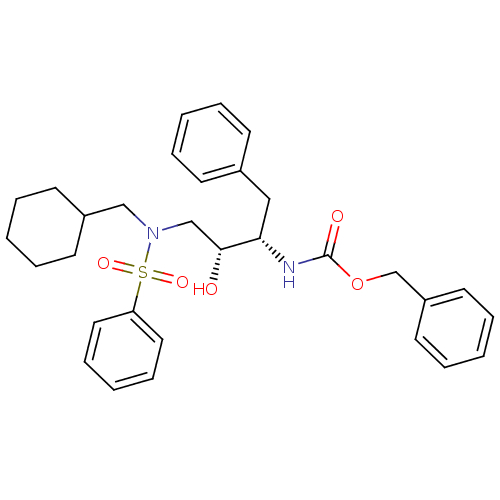 (CHEMBL348282 | [(1S,2R)-3-(Benzenesulfonyl-cyclohe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036253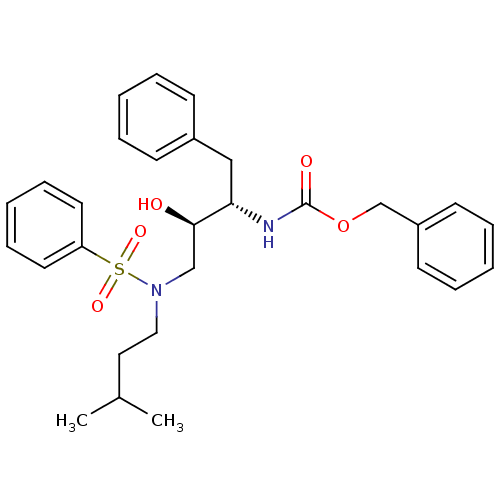 (CHEMBL347431 | {(1S,2R)-3-[Benzenesulfonyl-(3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036259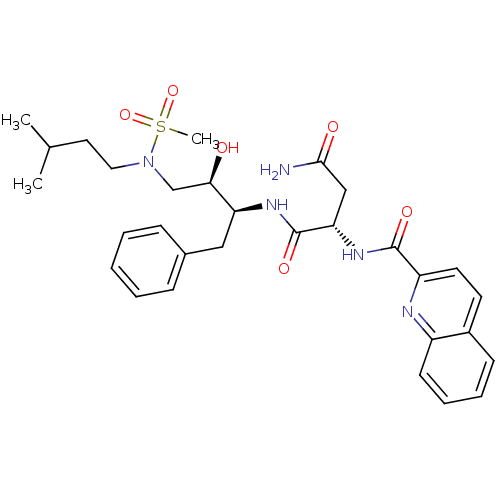 ((S)-N*1*-{(1S,2R)-1-Benzyl-2-hydroxy-3-[methanesul...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036258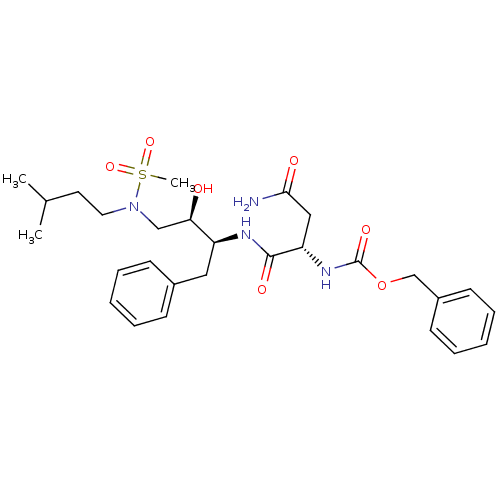 (((S)-1-{(1S,2R)-1-Benzyl-2-hydroxy-3-[methanesulfo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036260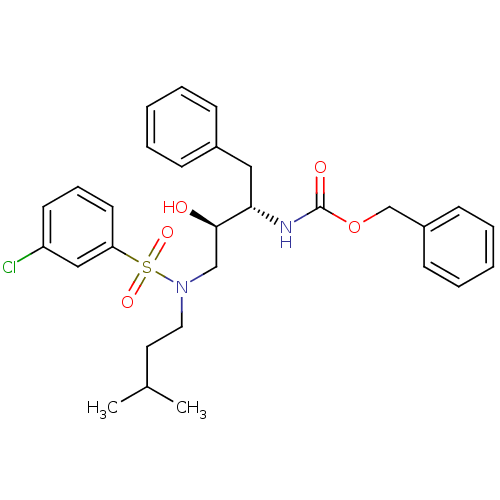 (CHEMBL156424 | {(1S,2R)-1-Benzyl-3-[(3-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036255 (CHEMBL154197 | [(1S,2R)-3-(Benzenesulfonyl-benzyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036254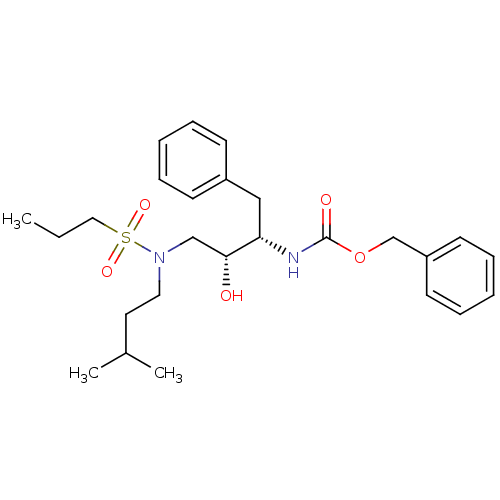 (CHEMBL155051 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(3-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036264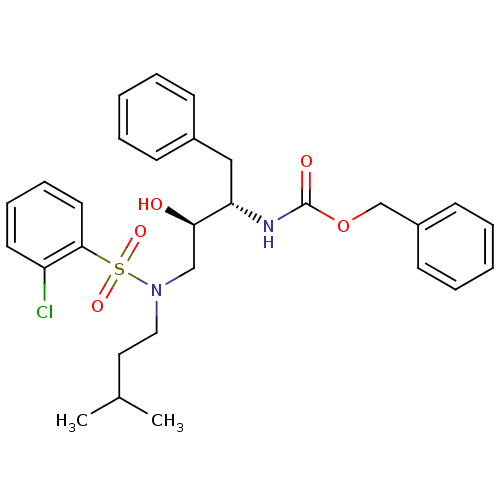 (CHEMBL154600 | {(1S,2R)-1-Benzyl-3-[(2-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036263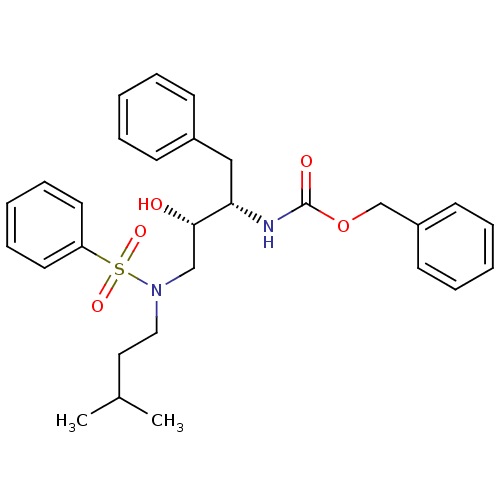 (CHEMBL154638 | {(1S,2S)-3-[Benzenesulfonyl-(3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406141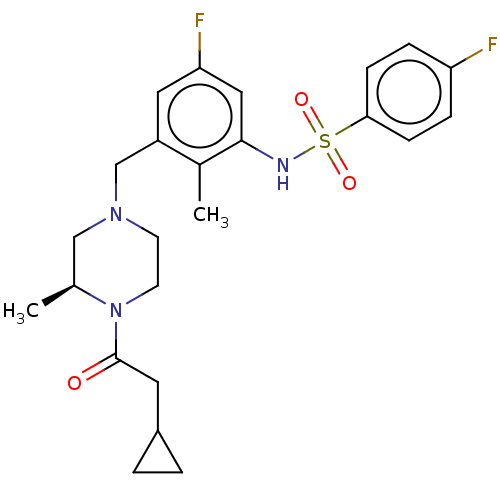 (CHEMBL5286547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036256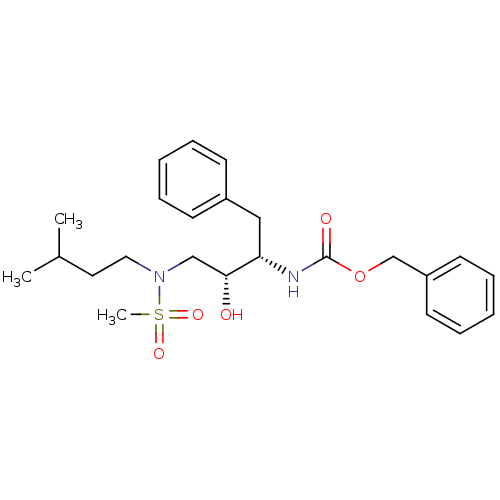 (CHEMBL154798 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406139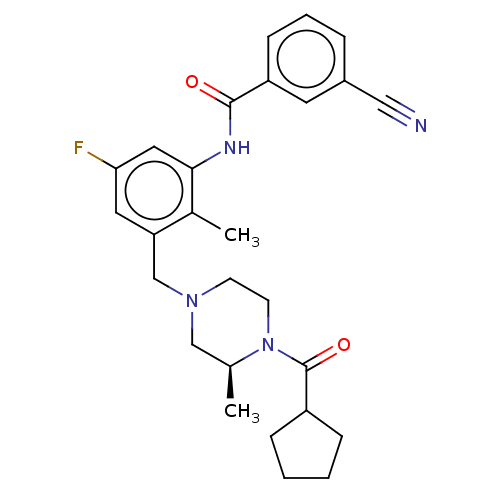 (CHEMBL5281816) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406140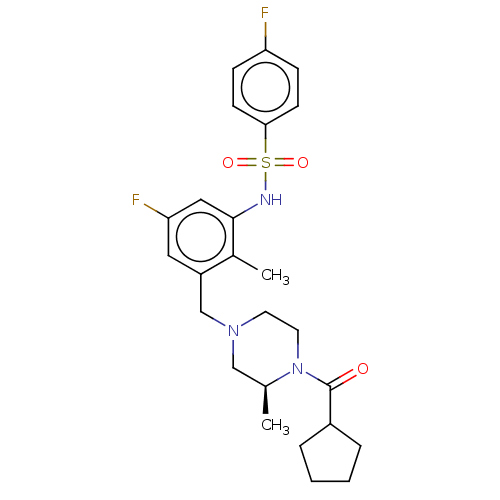 (CHEMBL5284779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406144 (CHEMBL5283506) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406145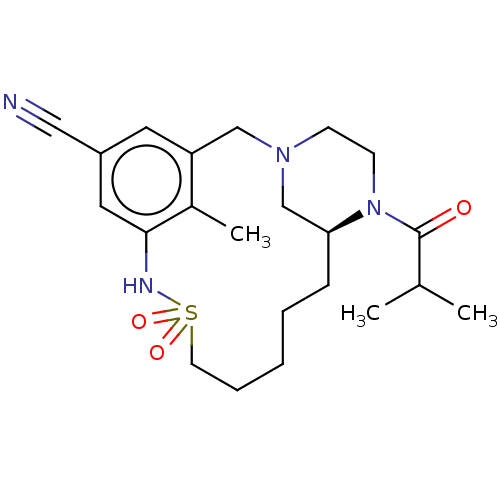 (CHEMBL5287800) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406143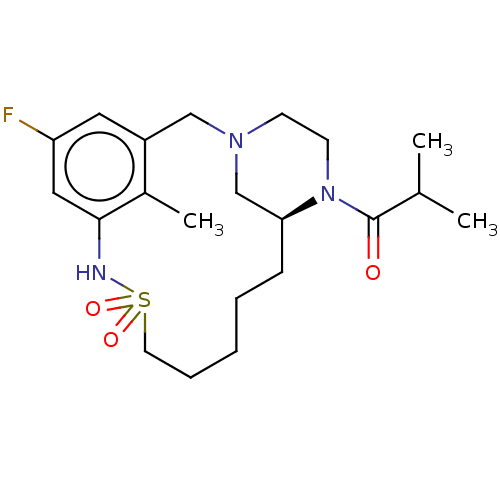 (CHEMBL5287916) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406142 (CHEMBL5280788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490009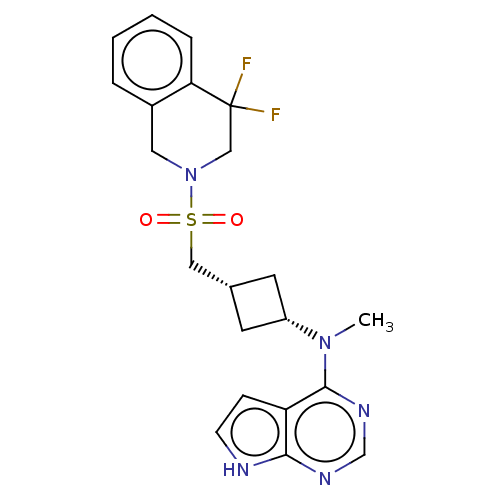 (US10966980, Example 263) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490011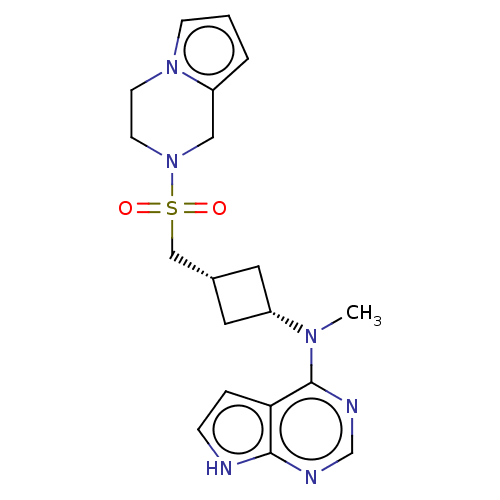 (US10966980, Example 265) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489983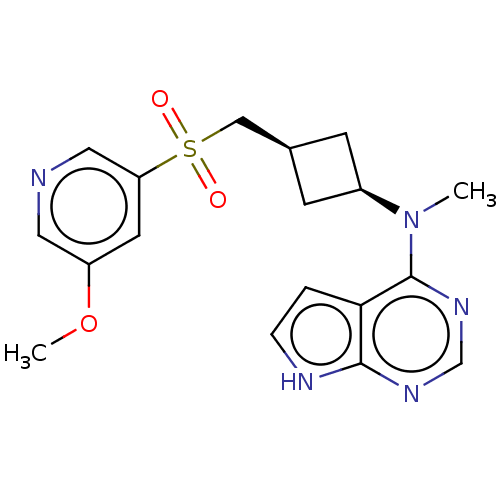 (US10966980, Example 237) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489965 (US10966980, Example 219) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489982 (US10966980, Example 236) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489909 (US10966980, Example 163) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489985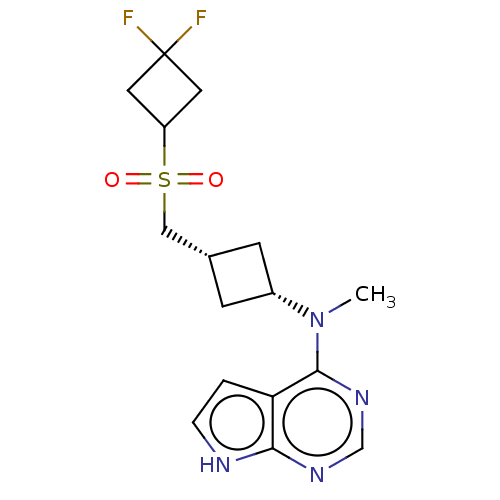 (US10966980, Example 239) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489998 (US10966980, Example 252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489960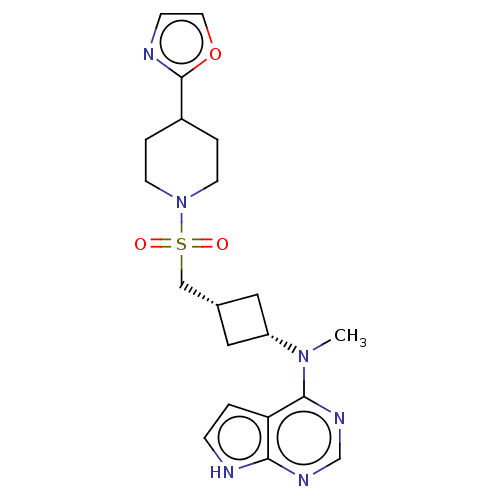 (US10966980, Example 214) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489922 (US10966980, Example 176) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490010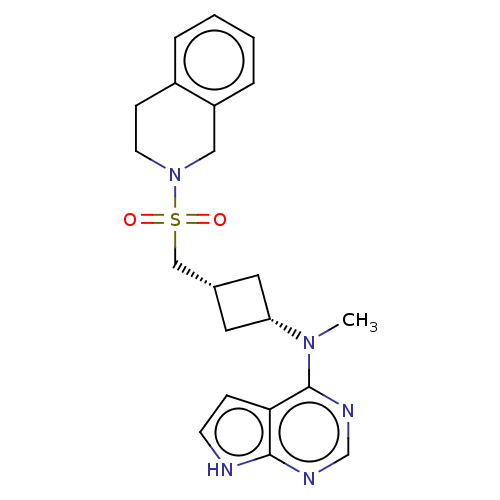 (US10966980, Example 264) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489986 (US10966980, Example 240) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489974 (US10966980, Example 228) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490006 (US10966980, Example 260) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489977 (US10966980, Example 231) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490008 (US10966980, Example 262) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489916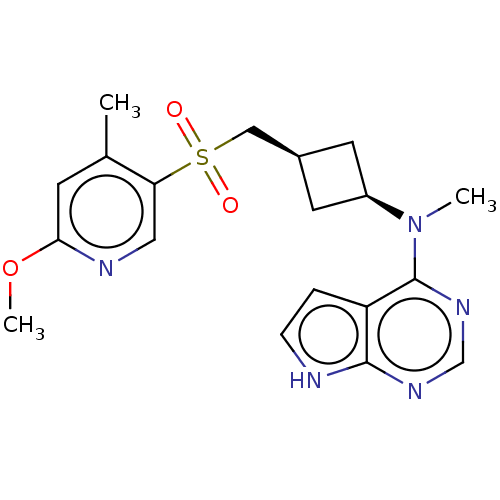 (US10966980, Example 170) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489940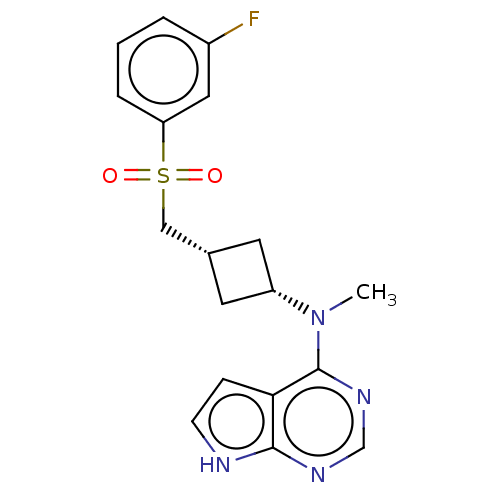 (US10966980, Example 194) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489987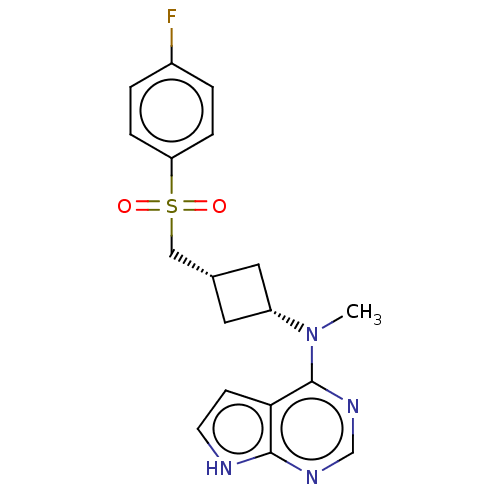 (US10966980, Example 241) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490005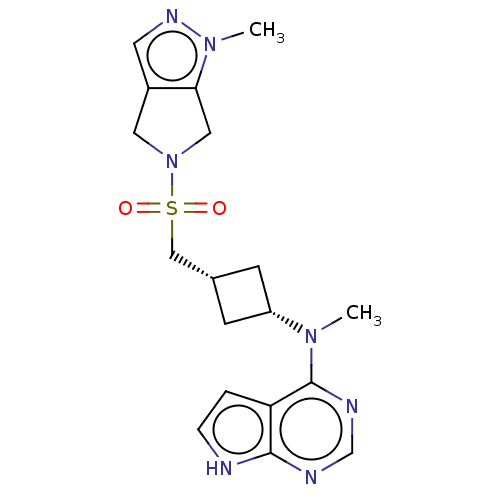 (US10966980, Example 259) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489978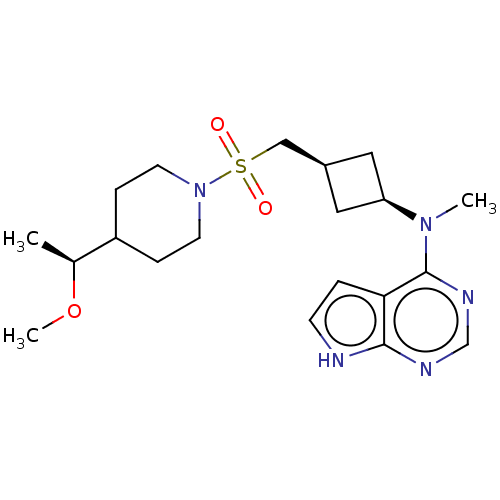 (US10966980, Example 232) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489941 (US10966980, Example 195) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489994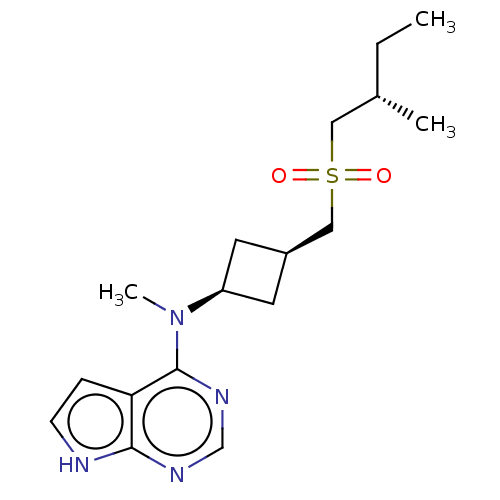 (US10966980, Example 248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489906 (US10966980, Example 160) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489968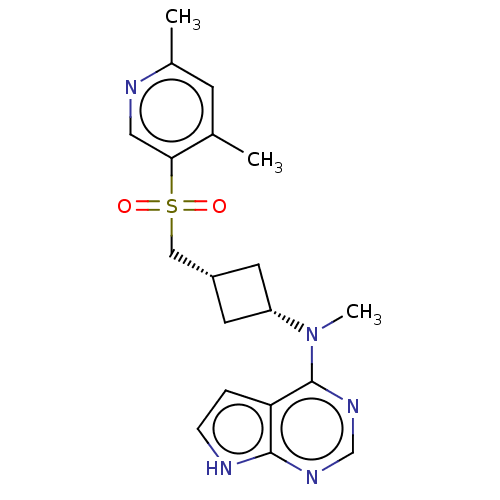 (US10966980, Example 222) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489934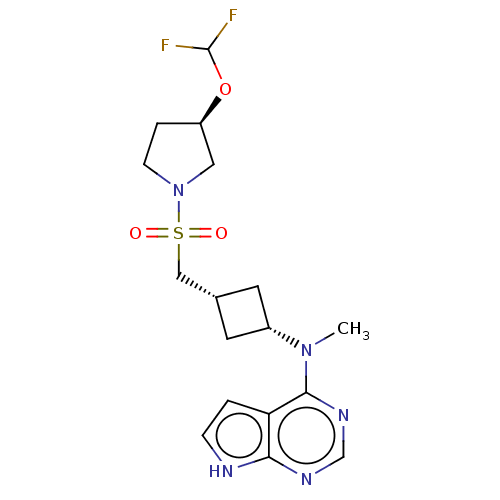 (US10966980, Example 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489970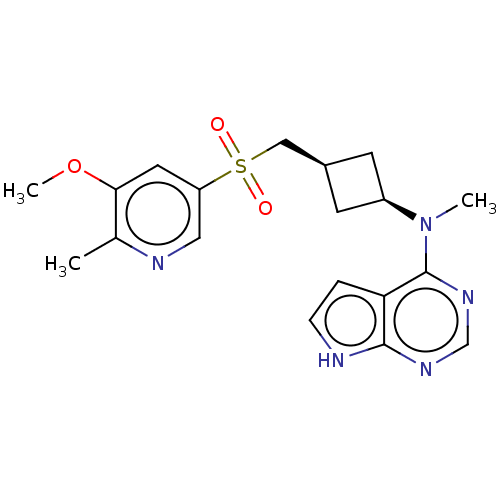 (US10966980, Example 224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489969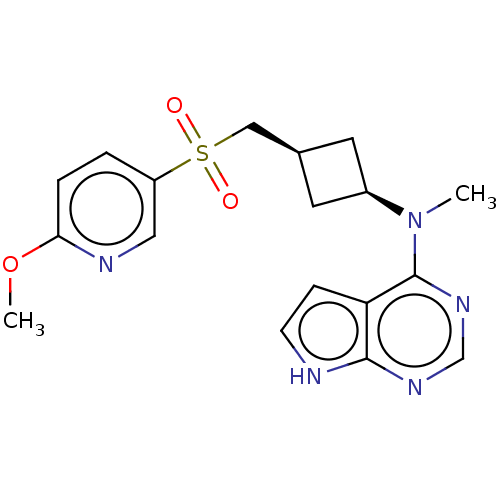 (US10966980, Example 223) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489901 (US10966980, Example 155) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2233 total ) | Next | Last >> |