

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127246 (8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of Geranylgeranylprotein transferase-I at 10 uM | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127246 (8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163592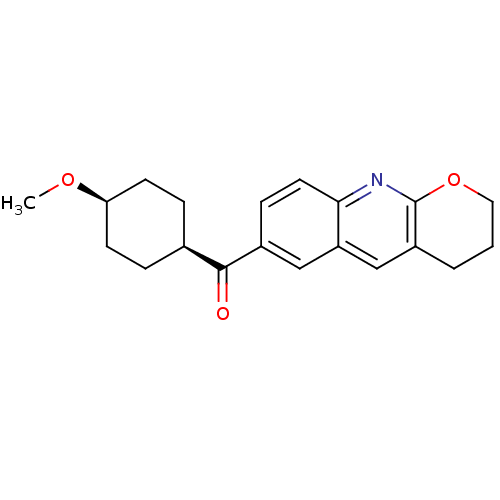 ((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163592 ((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163613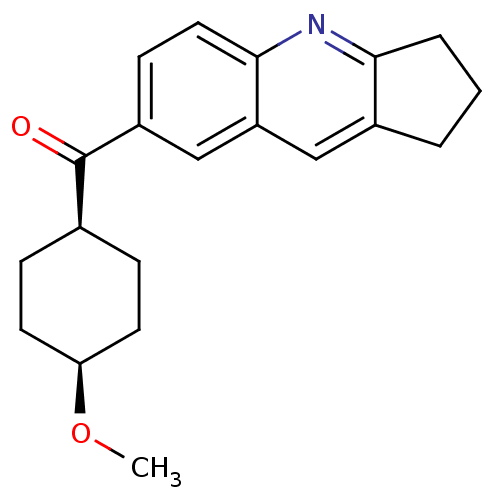 ((2,3-Dihydro-1H-cyclopenta[b]quinolin-7-yl)-(4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136383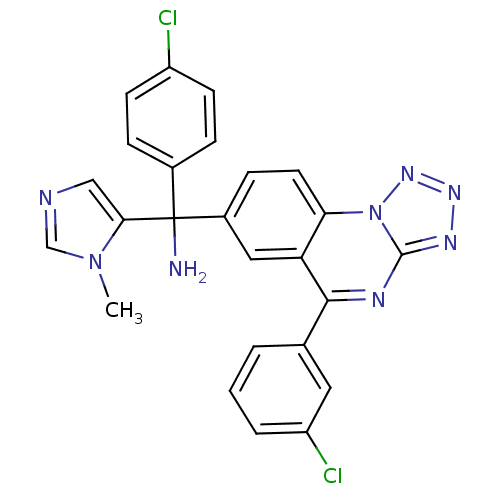 ((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136383 ((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50126335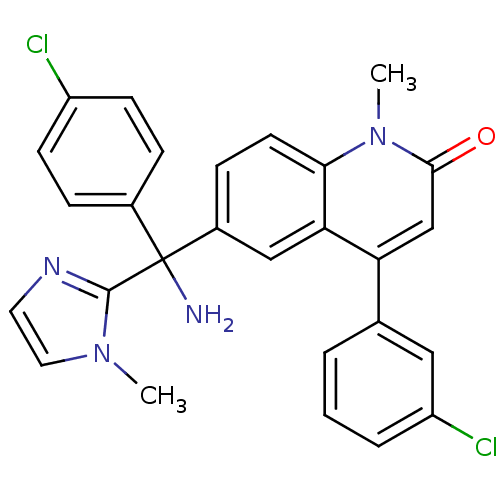 (6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14434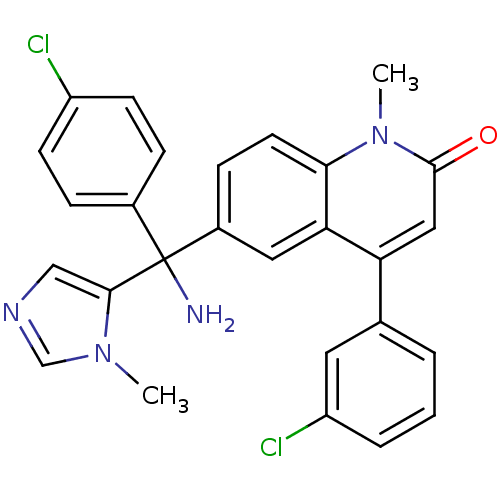 (6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Janssen Research Foundation | Assay Description Compounds were tested as inhibitors of FTase in vitro using purified human enzyme to catalyze the reaction between [3H] FPP and a biotinylated peptid... | Cancer Res 61: 131-7 (2001) BindingDB Entry DOI: 10.7270/Q20C4T2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50126335 (6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50126335 (6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136377 (4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-(4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127248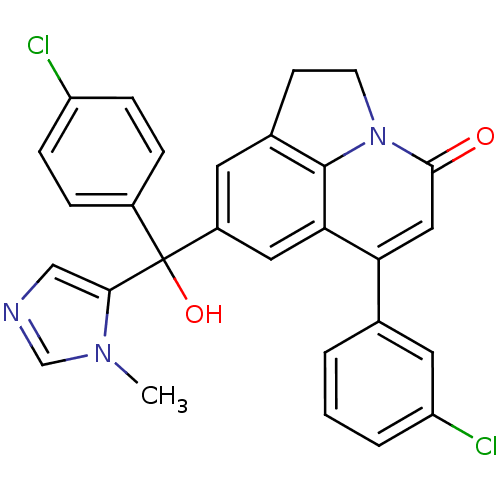 (6-(3-Chloro-phenyl)-8-[(4-chloro-phenyl)-hydroxy-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163596 ((2-Amino-3-ethyl-quinolin-6-yl)-(4-methoxy-cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136375 (6-[Amino-(4-chloro-phenyl)-(4-methyl-4H-[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136385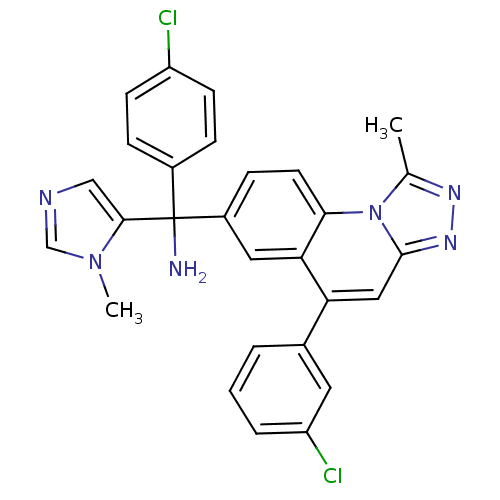 (C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-1-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127252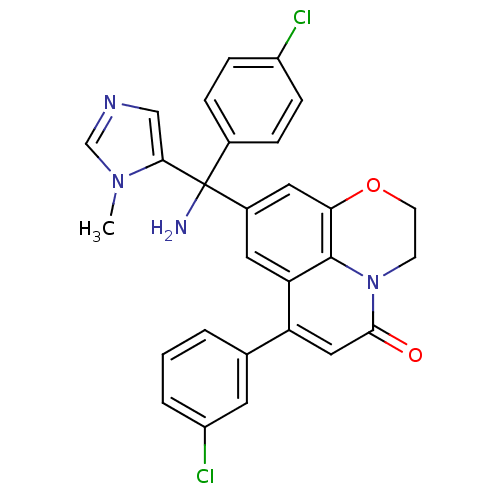 (8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127254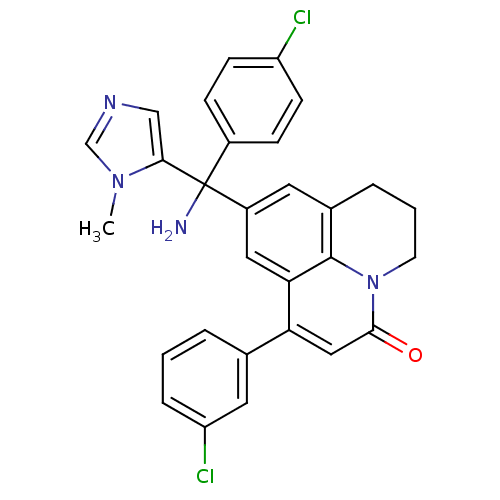 (9-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136379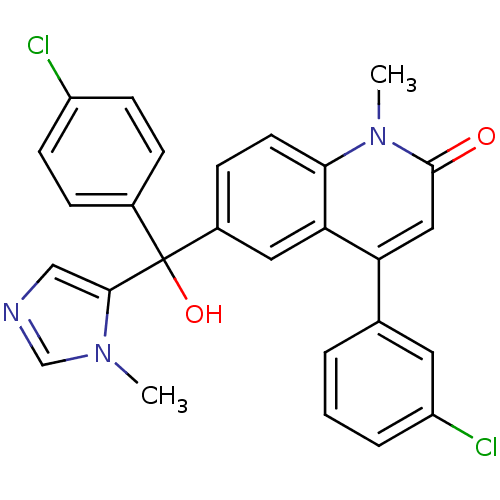 (4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136384 ((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136384 ((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163617 ((2,3-Dihydro-thieno[2,3-b]quinolin-6-yl)-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163617 ((2,3-Dihydro-thieno[2,3-b]quinolin-6-yl)-(4-methox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136376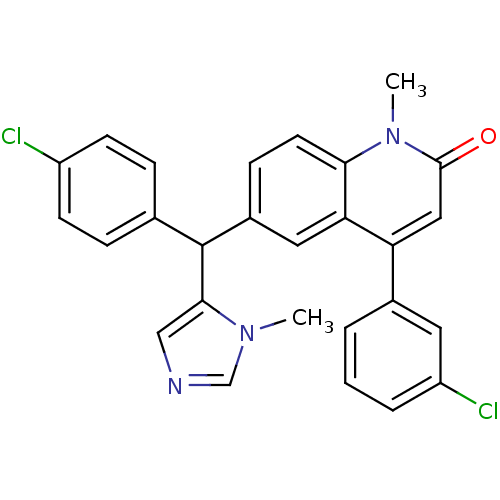 (4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-(3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163592 ((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163589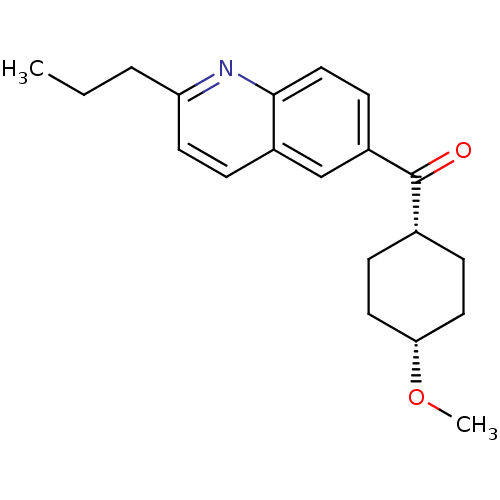 ((4-Methoxy-cyclohexyl)-(2-propyl-quinolin-6-yl)-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163592 ((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163590 ((2,3-Diethyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163590 ((2,3-Diethyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136389 (C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-[1,2,4]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136384 ((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127255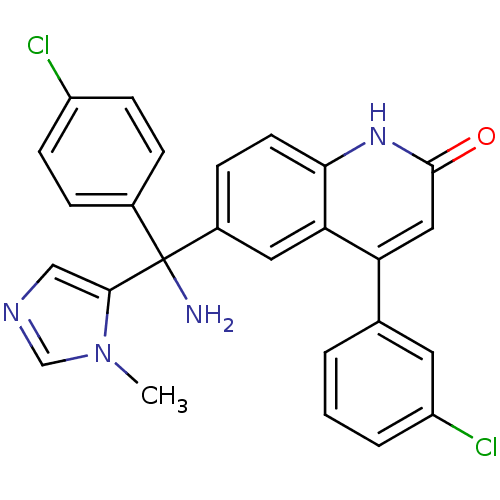 (6-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127247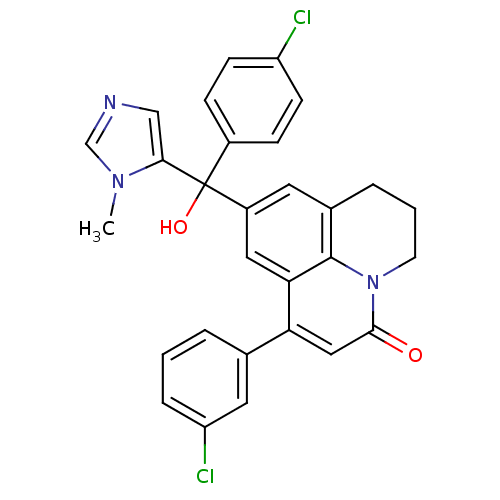 (1-(3-Chloro-phenyl)-9-[(4-chloro-phenyl)-hydroxy-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163613 ((2,3-Dihydro-1H-cyclopenta[b]quinolin-7-yl)-(4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136380 (4-(3-Chloro-phenyl)-6-[1-(4-chloro-phenyl)-1-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136378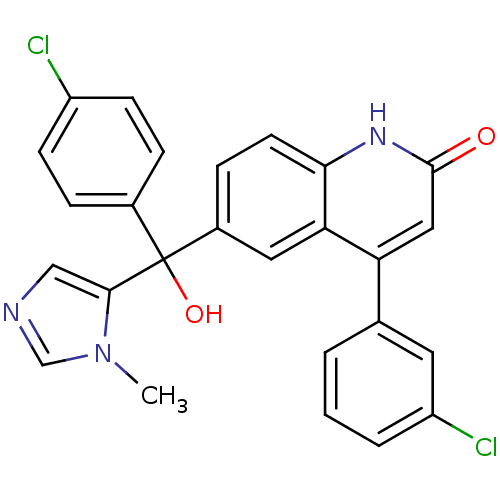 (4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163607 (3-ethyl-6-[(4-methoxycyclohexyl)carbonyl]quinoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163616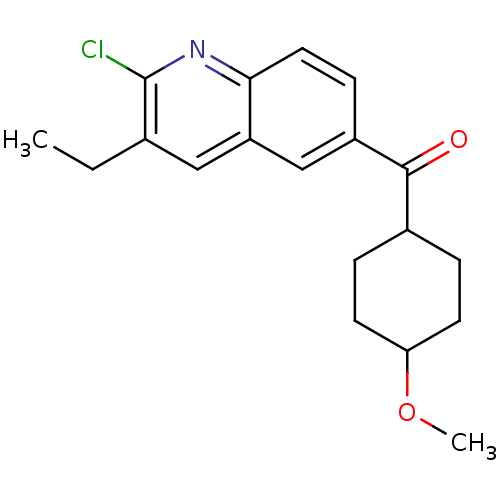 ((2-Chloro-3-ethyl-quinolin-6-yl)-(4-methoxy-cycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136386 (C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163616 ((2-Chloro-3-ethyl-quinolin-6-yl)-(4-methoxy-cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136382 (4-(3-Chloro-phenyl)-6-[1-(4-chloro-phenyl)-1-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163616 ((2-Chloro-3-ethyl-quinolin-6-yl)-(4-methoxy-cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163589 ((4-Methoxy-cyclohexyl)-(2-propyl-quinolin-6-yl)-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163609 ((4-Methoxy-cyclohexyl)-(2-methyl-3-propyl-quinolin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against human metabotropic glutamate receptor | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127249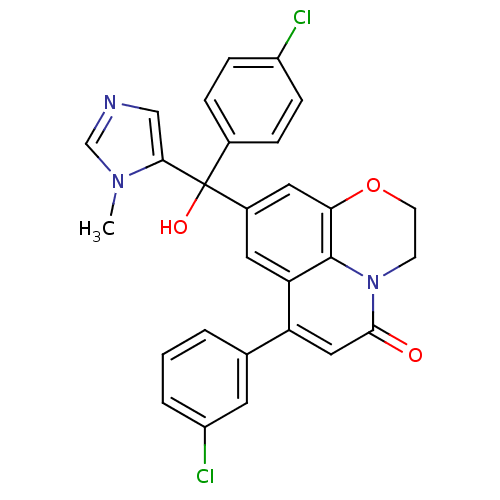 (6-(3-Chloro-phenyl)-8-[(4-chloro-phenyl)-hydroxy-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127257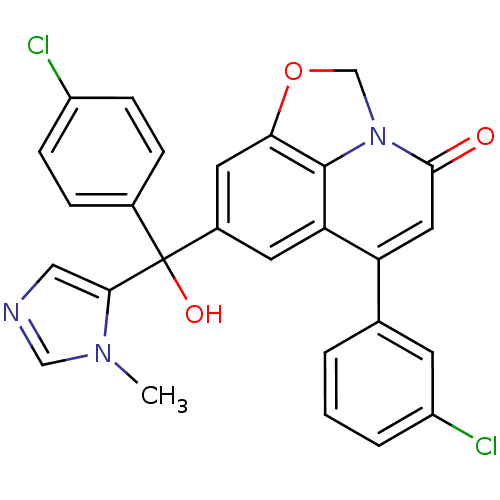 (6-(3-Chloro-phenyl)-8-[(4-chloro-phenyl)-hydroxy-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136381 (4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4361-4 (2003) BindingDB Entry DOI: 10.7270/Q25H7FPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163606 (1-(3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-2-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibitory concentration against rat metabotropic glutamate receptor 1 | J Med Chem 48: 2134-53 (2005) Article DOI: 10.1021/jm049499o BindingDB Entry DOI: 10.7270/Q2RR2017 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127250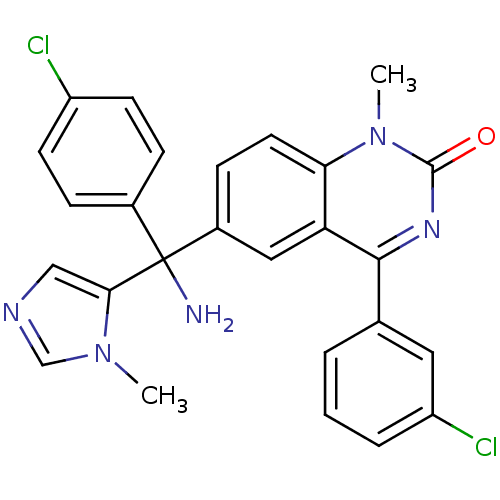 (6-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136387 (C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 125 total ) | Next | Last >> |