

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116042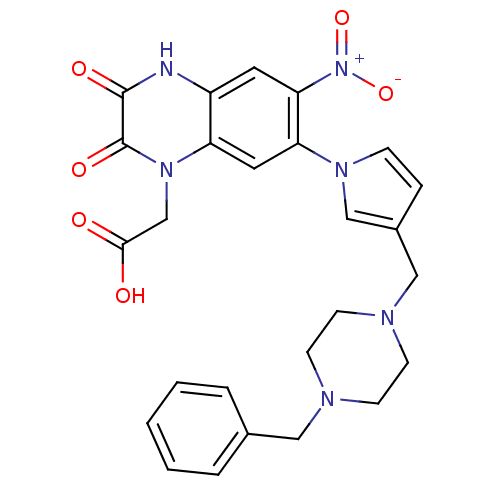 (CHEMBL63861 | {7-[3-(4-Benzyl-piperazin-1-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50128678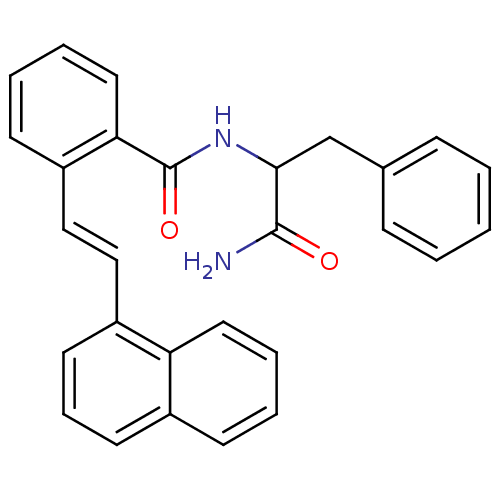 (CHEMBL310855 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116048 (CHEMBL67828 | {6-Nitro-2,3-dioxo-7-[3-(4-phenethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50128679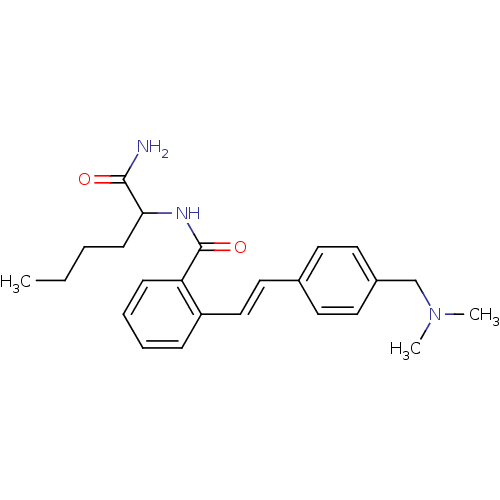 (CHEMBL77784 | N-(1-Carbamoyl-pentyl)-2-[2-(4-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50128680 (CHEMBL80903 | N-(1-Benzyl-2-oxo-ethyl)-2-(2-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50073850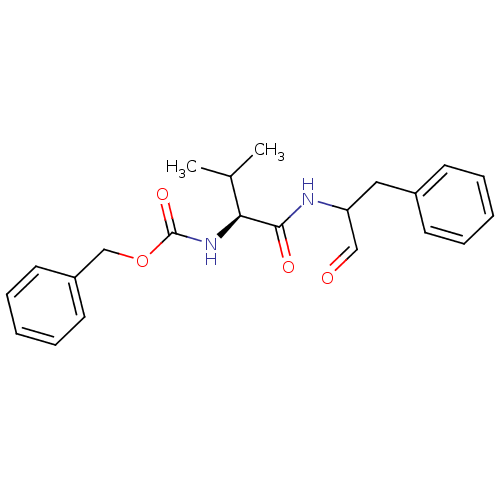 ((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50073850 ((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of cathepsin B | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50128681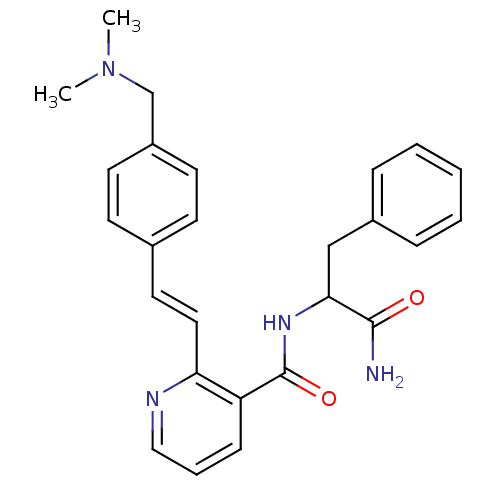 (CHEMBL80933 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50128679 (CHEMBL77784 | N-(1-Carbamoyl-pentyl)-2-[2-(4-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of cathepsin B | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50128682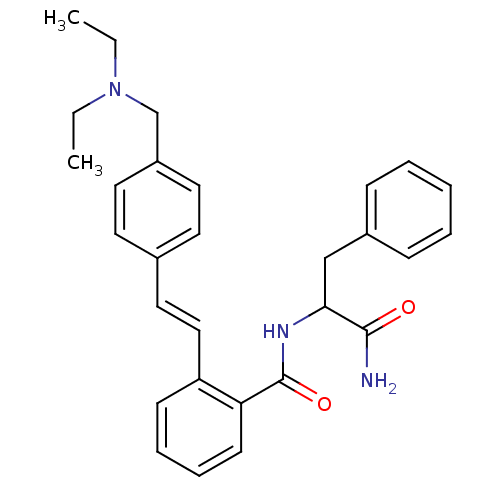 (CHEMBL80605 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116040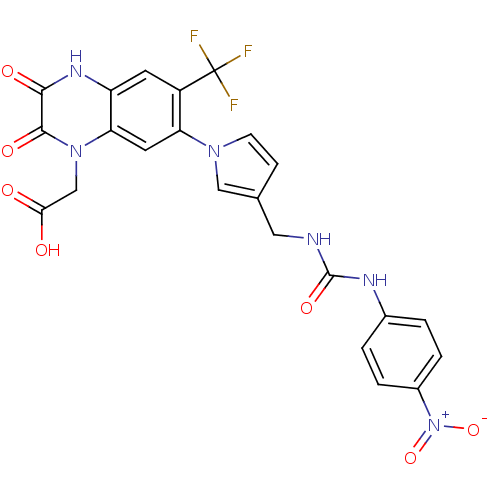 ((7-{3-[3-(4-Nitro-phenyl)-ureidomethyl]-pyrrol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50128682 (CHEMBL80605 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of cathepsin B | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50128681 (CHEMBL80933 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of cathepsin B | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50128678 (CHEMBL310855 | N-(1-Carbamoyl-2-phenyl-ethyl)-2-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of cathepsin B | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116040 ((7-{3-[3-(4-Nitro-phenyl)-ureidomethyl]-pyrrol-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112839 (CHEMBL26631 | N-(1-Benzyl-2-oxo-ethyl)-2-styryl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116046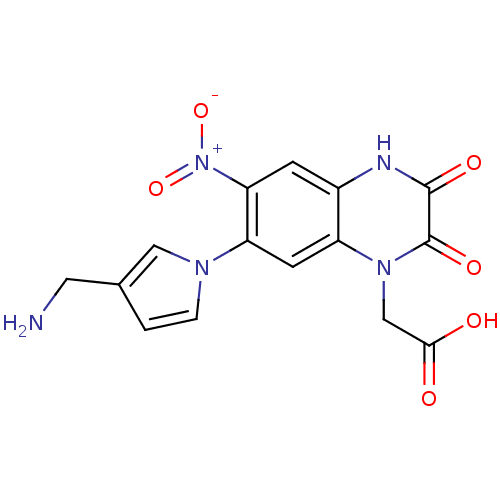 (CHEMBL12465 | [7-(3-Aminomethyl-pyrrol-1-yl)-6-nit...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116044 (CHEMBL416685 | {6-Nitro-2,3-dioxo-7-[3-(4-phenyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116047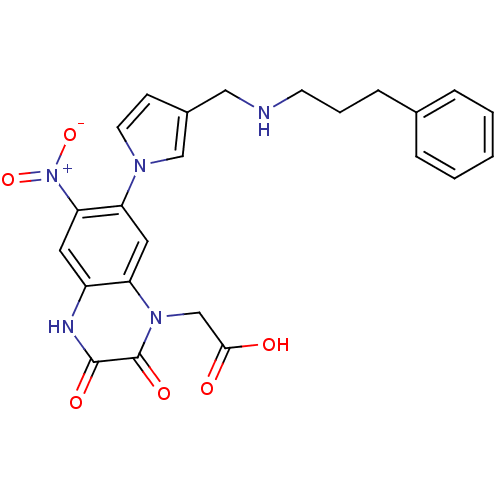 ((6-Nitro-2,3-dioxo-7-{3-[(3-phenyl-propylamino)-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116041 (CHEMBL303415 | [6-Nitro-2,3-dioxo-7-(3-piperidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116045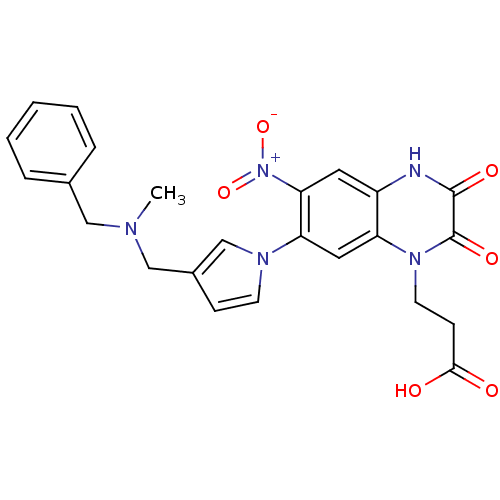 (3-(7-{3-[(Benzyl-methyl-amino)-methyl]-pyrrol-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116049 ((6-Nitro-2,3-dioxo-7-pyrrol-1-yl-3,4-dihydro-2H-qu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116044 (CHEMBL416685 | {6-Nitro-2,3-dioxo-7-[3-(4-phenyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116040 ((7-{3-[3-(4-Nitro-phenyl)-ureidomethyl]-pyrrol-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116042 (CHEMBL63861 | {7-[3-(4-Benzyl-piperazin-1-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112838 (CHEMBL281874 | N-(1-Benzyl-2-oxo-ethyl)-benzamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human Calpain 1 isolated from erythrocytes | J Med Chem 46: 2404-12 (2003) Article DOI: 10.1021/jm0210717 BindingDB Entry DOI: 10.7270/Q28P5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116043 (3-(6-Nitro-2,3-dioxo-7-pyrrol-1-yl-3,4-dihydro-2H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116049 ((6-Nitro-2,3-dioxo-7-pyrrol-1-yl-3,4-dihydro-2H-qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human Kai-2 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116048 (CHEMBL67828 | {6-Nitro-2,3-dioxo-7-[3-(4-phenethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116050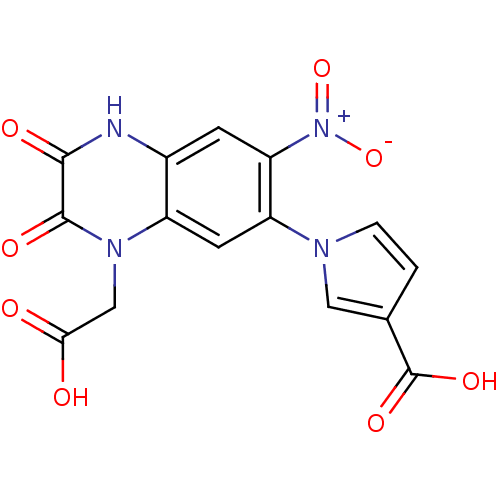 (1-(4-Carboxymethyl-7-nitro-2,3-dioxo-1,2,3,4-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116047 ((6-Nitro-2,3-dioxo-7-{3-[(3-phenyl-propylamino)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116048 (CHEMBL67828 | {6-Nitro-2,3-dioxo-7-[3-(4-phenethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116046 (CHEMBL12465 | [7-(3-Aminomethyl-pyrrol-1-yl)-6-nit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50116043 (3-(6-Nitro-2,3-dioxo-7-pyrrol-1-yl-3,4-dihydro-2H-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116043 (3-(6-Nitro-2,3-dioxo-7-pyrrol-1-yl-3,4-dihydro-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116044 (CHEMBL416685 | {6-Nitro-2,3-dioxo-7-[3-(4-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116049 ((6-Nitro-2,3-dioxo-7-pyrrol-1-yl-3,4-dihydro-2H-qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116042 (CHEMBL63861 | {7-[3-(4-Benzyl-piperazin-1-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116050 (1-(4-Carboxymethyl-7-nitro-2,3-dioxo-1,2,3,4-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM50116041 (CHEMBL303415 | [6-Nitro-2,3-dioxo-7-(3-piperidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116050 (1-(4-Carboxymethyl-7-nitro-2,3-dioxo-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116047 ((6-Nitro-2,3-dioxo-7-{3-[(3-phenyl-propylamino)-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116046 (CHEMBL12465 | [7-(3-Aminomethyl-pyrrol-1-yl)-6-nit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined towards Kai-2 using [3]H-kainate as the radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116050 (1-(4-Carboxymethyl-7-nitro-2,3-dioxo-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined towards Kai-2 using [3]H-kainate as the radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116040 ((7-{3-[3-(4-Nitro-phenyl)-ureidomethyl]-pyrrol-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity of the compound was determined towards Kai-2 using [3]H-kainate as the radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116044 (CHEMBL416685 | {6-Nitro-2,3-dioxo-7-[3-(4-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human Kai-2 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116042 (CHEMBL63861 | {7-[3-(4-Benzyl-piperazin-1-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human Kai-2 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116047 ((6-Nitro-2,3-dioxo-7-{3-[(3-phenyl-propylamino)-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human Kai-2 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50116045 (3-(7-{3-[(Benzyl-methyl-amino)-methyl]-pyrrol-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainate | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50116048 (CHEMBL67828 | {6-Nitro-2,3-dioxo-7-[3-(4-phenethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity towards cloned human Kai-2 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligand | Bioorg Med Chem Lett 12: 2113-6 (2002) BindingDB Entry DOI: 10.7270/Q22806XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |