 Found 48 hits with Last Name = 'vo' and Initial = 'dd'
Found 48 hits with Last Name = 'vo' and Initial = 'dd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066791
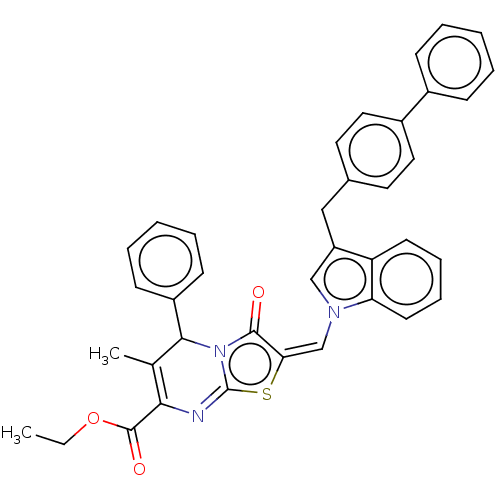
(CHEMBL3400492)Show SMILES CCOC(=O)C1=C(C)C(c2ccccc2)n2c(=N1)s\c(=C\n1cc(Cc3ccc(cc3)-c3ccccc3)c3ccccc13)c2=O |c:5,17| Show InChI InChI=1S/C38H31N3O3S/c1-3-44-37(43)34-25(2)35(29-14-8-5-9-15-29)41-36(42)33(45-38(41)39-34)24-40-23-30(31-16-10-11-17-32(31)40)22-26-18-20-28(21-19-26)27-12-6-4-7-13-27/h4-21,23-24,35H,3,22H2,1-2H3/b33-24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50520046

(CHEMBL1340426)Show InChI InChI=1S/C13H10N4O2/c14-13-15-11(9-5-2-1-3-6-9)16-17(13)12(18)10-7-4-8-19-10/h1-8H,(H2,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1A receptor expressed in human CHO cell membranes incubated for 60 mins by scintillation counting met... |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50181876
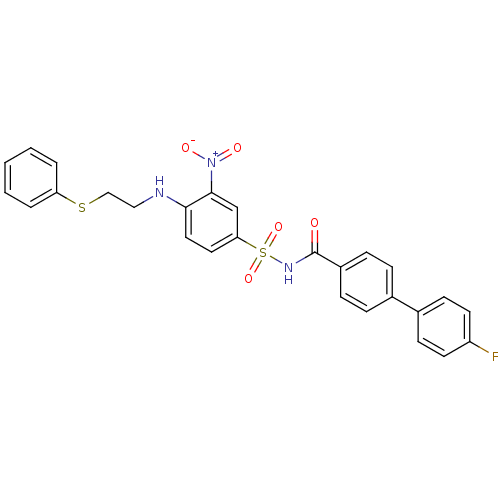
(CHEMBL371861 | N-(4'-fluoro-biphenyl-4-carbonyl)-3...)Show SMILES [O-][N+](=O)c1cc(ccc1NCCSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H22FN3O5S2/c28-22-12-10-20(11-13-22)19-6-8-21(9-7-19)27(32)30-38(35,36)24-14-15-25(26(18-24)31(33)34)29-16-17-37-23-4-2-1-3-5-23/h1-15,18,29H,16-17H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50520046

(CHEMBL1340426)Show InChI InChI=1S/C13H10N4O2/c14-13-15-11(9-5-2-1-3-6-9)16-17(13)12(18)10-7-4-8-19-10/h1-8H,(H2,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from adenosine A2A receptor in human HeLa cell membranes incubated for 30 mins by scintillation counting method |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50520047

(CHEMBL1465169)Show InChI InChI=1S/C20H28N2O2/c1-15-6-8-16(9-7-15)20(24)17-10-12-22(13-11-17)14-19(23)21-18-4-2-3-5-18/h6-9,17-18H,2-5,10-14H2,1H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human dopamine D4 receptor expressed in cell membranes incubated for 60 mins by scintillation counting method |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066787

(CHEMBL1374578)Show SMILES OC(=O)c1ccccc1C(=O)c1ccc(Sc2ccc(Cl)cc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C20H12ClNO5S/c21-13-6-8-14(9-7-13)28-18-10-5-12(11-17(18)22(26)27)19(23)15-3-1-2-4-16(15)20(24)25/h1-11H,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50520044
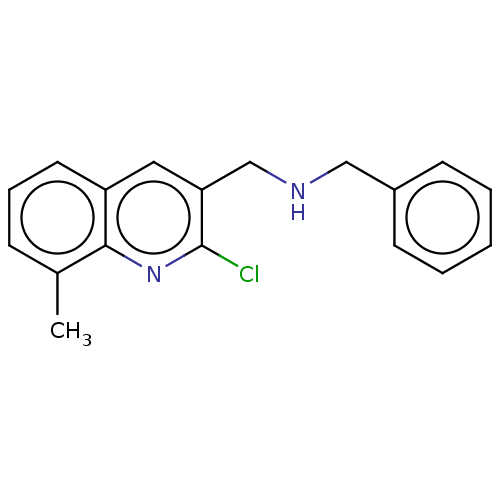
(CHEMBL1406529)Show InChI InChI=1S/C18H17ClN2/c1-13-6-5-9-15-10-16(18(19)21-17(13)15)12-20-11-14-7-3-2-4-8-14/h2-10,20H,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human dopamine D4 receptor expressed in cell membranes incubated for 60 mins by scintillation counting method |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066792

(CHEMBL3400491)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)N[C@@H](CC(O)=O)Cc3cccc4ccccc34)c2c1 |r| Show InChI InChI=1S/C33H29ClN2O5/c1-20-28(29-18-26(41-2)14-15-30(29)36(20)33(40)22-10-12-24(34)13-11-22)19-31(37)35-25(17-32(38)39)16-23-8-5-7-21-6-3-4-9-27(21)23/h3-15,18,25H,16-17,19H2,1-2H3,(H,35,37)(H,38,39)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM23223
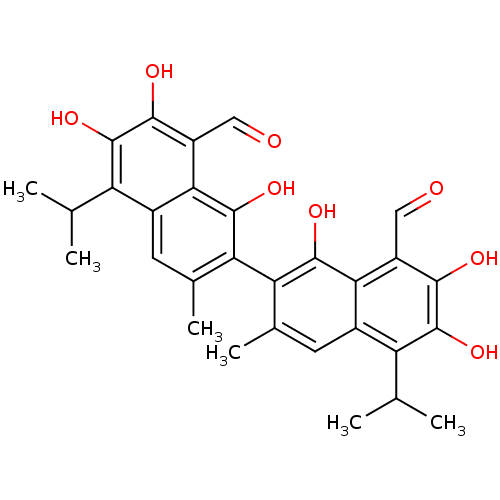
(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066786

(CHEMBL3400494)Show SMILES CC(C)(C)c1ccccc1Cc1cc(C(=O)Nc2ccc(cc2)S(=O)(=O)c2ccccc2C(C)(C)C)c(O)c(O)c1O Show InChI InChI=1S/C34H37NO6S/c1-33(2,3)26-12-8-7-11-21(26)19-22-20-25(30(37)31(38)29(22)36)32(39)35-23-15-17-24(18-16-23)42(40,41)28-14-10-9-13-27(28)34(4,5)6/h7-18,20,36-38H,19H2,1-6H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50520044

(CHEMBL1406529)Show InChI InChI=1S/C18H17ClN2/c1-13-6-5-9-15-10-16(18(19)21-17(13)15)12-20-11-14-7-3-2-4-8-14/h2-10,20H,11-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human dopamine D3 receptor expressed in cell membranes incubated for 60 mins by scintillation counting method |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50520045
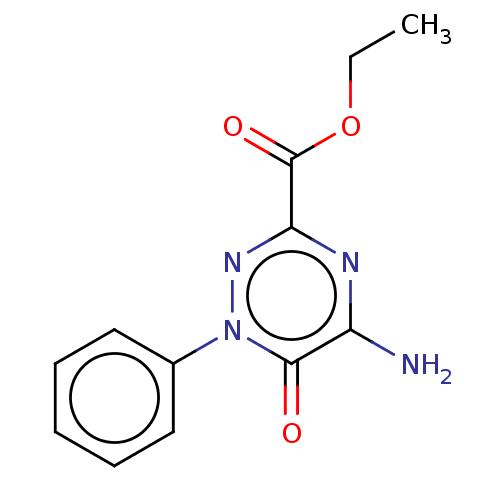
(CHEMBL1712711)Show InChI InChI=1S/C12H12N4O3/c1-2-19-12(18)10-14-9(13)11(17)16(15-10)8-6-4-3-5-7-8/h3-7H,2H2,1H3,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1A receptor expressed in human CHO cell membranes incubated for 60 mins by scintillation counting met... |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50107130
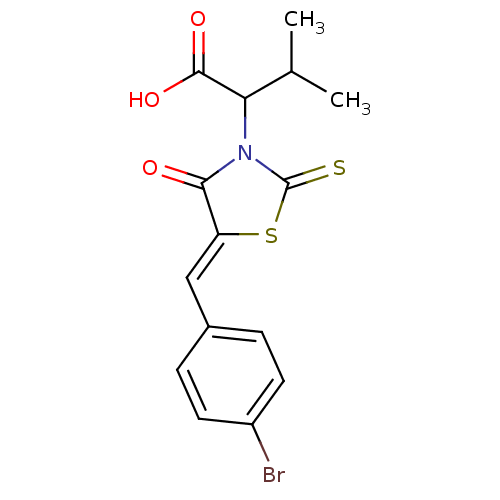
((Z)-2-(5-(4-bromobenzylidene)-4-oxo-2-thioxothiazo...)Show SMILES CC(C)C(N1C(=S)S\C(=C/c2ccc(Br)cc2)C1=O)C(O)=O Show InChI InChI=1S/C15H14BrNO3S2/c1-8(2)12(14(19)20)17-13(18)11(22-15(17)21)7-9-3-5-10(16)6-4-9/h3-8,12H,1-2H3,(H,19,20)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066790
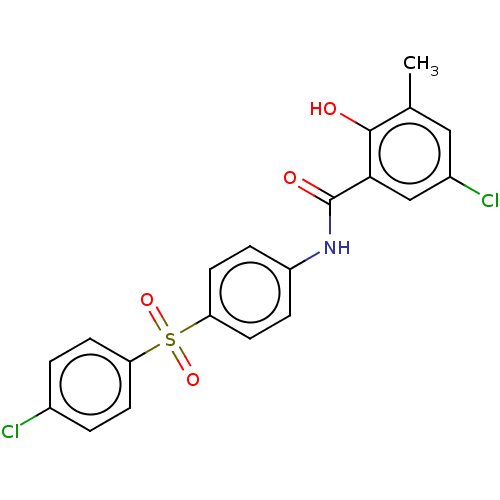
(CHEMBL3400493)Show SMILES Cc1cc(Cl)cc(C(=O)Nc2ccc(cc2)S(=O)(=O)c2ccc(Cl)cc2)c1O Show InChI InChI=1S/C20H15Cl2NO4S/c1-12-10-14(22)11-18(19(12)24)20(25)23-15-4-8-17(9-5-15)28(26,27)16-6-2-13(21)3-7-16/h2-11,24H,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50520045

(CHEMBL1712711)Show InChI InChI=1S/C12H12N4O3/c1-2-19-12(18)10-14-9(13)11(17)16(15-10)8-6-4-3-5-7-8/h3-7H,2H2,1H3,(H2,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from adenosine A2A receptor in human HeLa cell membranes incubated for 30 mins by scintillation counting method |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50520047

(CHEMBL1465169)Show InChI InChI=1S/C20H28N2O2/c1-15-6-8-16(9-7-15)20(24)17-10-12-22(13-11-17)14-19(23)21-18-4-2-3-5-18/h6-9,17-18H,2-5,10-14H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human dopamine D3 receptor expressed in cell membranes incubated for 60 mins by scintillation counting method |
J Med Chem 63: 613-620 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01560
BindingDB Entry DOI: 10.7270/Q2WQ076H |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Rennes1
Curated by ChEMBL
| Assay Description
Binding affinity to Mcl-1 |
Eur J Med Chem 51: 286-93 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.036
BindingDB Entry DOI: 10.7270/Q2K64K2D |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50388980
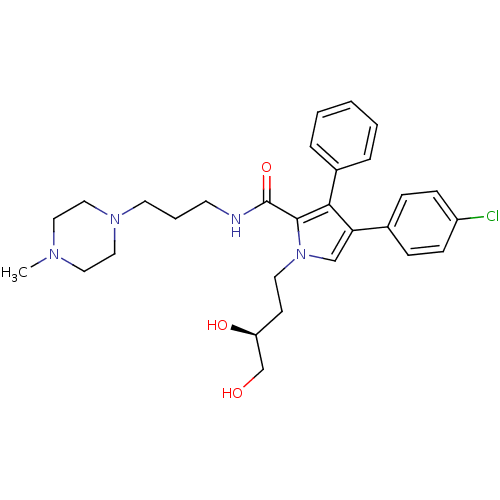
(CHEMBL2063880)Show SMILES CN1CCN(CCCNC(=O)c2c(c(cn2CC[C@H](O)CO)-c2ccc(Cl)cc2)-c2ccccc2)CC1 |r| Show InChI InChI=1S/C29H37ClN4O3/c1-32-16-18-33(19-17-32)14-5-13-31-29(37)28-27(23-6-3-2-4-7-23)26(22-8-10-24(30)11-9-22)20-34(28)15-12-25(36)21-35/h2-4,6-11,20,25,35-36H,5,12-19,21H2,1H3,(H,31,37)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066761
(CHEMBL3400497)Show SMILES OC(=O)c1nc(sc1CCCCc1ccccc1)-c1ccc2CCC\C(=N/Nc3nc4ccccc4s3)c2c1 Show InChI InChI=1S/C31H28N4O2S2/c36-30(37)28-27(16-6-4-11-20-9-2-1-3-10-20)38-29(33-28)22-18-17-21-12-8-14-24(23(21)19-22)34-35-31-32-25-13-5-7-15-26(25)39-31/h1-3,5,7,9-10,13,15,17-19H,4,6,8,11-12,14,16H2,(H,32,35)(H,36,37)/b34-24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030764
(CHEMBL3342187)Show SMILES NCc1ccc(OCCCc2sc(nc2C(O)=O)-c2ccc3CCC\C(=N/Nc4nc5ccccc5s4)c3c2)cc1 Show InChI InChI=1S/C31H29N5O3S2/c32-18-19-10-14-22(15-11-19)39-16-4-9-27-28(30(37)38)34-29(40-27)21-13-12-20-5-3-7-24(23(20)17-21)35-36-31-33-25-6-1-2-8-26(25)41-31/h1-2,6,8,10-15,17H,3-5,7,9,16,18,32H2,(H,33,36)(H,37,38)/b35-24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50339777
((R)-N-(7-(4-((4'-chlorobiphenyl-2-yl)methyl)pipera...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)Nc1ncnc2cc(ccc12)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C43H45ClN8O4S2/c1-49(2)21-20-34(29-57-36-9-4-3-5-10-36)47-40-19-17-37(27-42(40)52(53)54)58(55,56)48-43-39-18-16-35(26-41(39)45-30-46-43)51-24-22-50(23-25-51)28-32-8-6-7-11-38(32)31-12-14-33(44)15-13-31/h3-19,26-27,30,34,47H,20-25,28-29H2,1-2H3,(H,45,46,48)/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50448452
(CHEMBL3126119)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(c(C)c1)-c1cccc2c(CCCOc3cccc4ccccc34)c(nn12)C(O)=O |r| Show InChI InChI=1S/C47H46N6O8S2/c1-31-28-33(46(54)50-63(59,60)36-22-24-40(43(29-36)53(57)58)48-34(25-26-51(2)3)30-62-35-14-5-4-6-15-35)21-23-37(31)41-18-10-19-42-39(45(47(55)56)49-52(41)42)17-11-27-61-44-20-9-13-32-12-7-8-16-38(32)44/h4-10,12-16,18-24,28-29,34,48H,11,17,25-27,30H2,1-3H3,(H,50,54)(H,55,56)/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066765
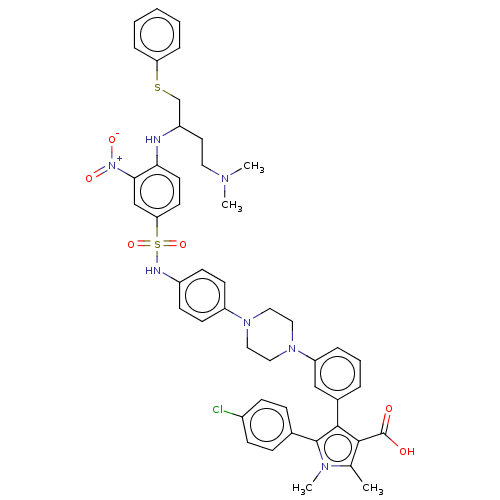
(CHEMBL3400495)Show SMILES CN(C)CCC(CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)Nc1ccc(cc1)N1CCN(CC1)c1cccc(c1)-c1c(C(O)=O)c(C)n(C)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C47H50ClN7O6S2/c1-32-44(47(56)57)45(46(52(32)4)33-13-15-35(48)16-14-33)34-9-8-10-39(29-34)54-27-25-53(26-28-54)38-19-17-36(18-20-38)50-63(60,61)41-21-22-42(43(30-41)55(58)59)49-37(23-24-51(2)3)31-62-40-11-6-5-7-12-40/h5-22,29-30,37,49-50H,23-28,31H2,1-4H3,(H,56,57) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
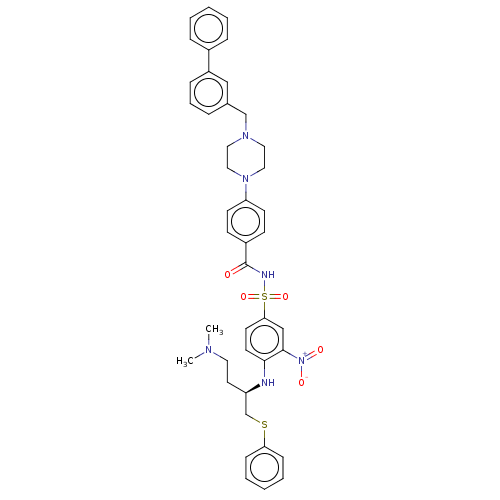
(Homo sapiens (Human)) | BDBM50066763
(CHEMBL1236741)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2cccc(c2)-c2ccccc2)CC1 |r| Show InChI InChI=1S/C42H46N6O5S2/c1-45(2)23-22-36(31-54-38-14-7-4-8-15-38)43-40-21-20-39(29-41(40)48(50)51)55(52,53)44-42(49)34-16-18-37(19-17-34)47-26-24-46(25-27-47)30-32-10-9-13-35(28-32)33-11-5-3-6-12-33/h3-21,28-29,36,43H,22-27,30-31H2,1-2H3,(H,44,49)/t36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50384326

(CHEMBL2030861)Show SMILES CCCCc1c(c(CO)nn1-c1ccccc1)-c1ccc(cc1C(=O)N1CCc2ccccc2C1)C(=O)NS(=O)(=O)CC[Si](C)(C)C |(33.41,-39.78,;34.7,-40.62,;36.07,-39.92,;36.15,-38.39,;37.53,-37.68,;38.96,-37.09,;38.83,-35.56,;40,-34.56,;39.72,-33.04,;37.33,-35.2,;36.53,-36.51,;35,-36.53,;34.22,-35.2,;32.68,-35.21,;31.92,-36.55,;32.71,-37.88,;34.24,-37.86,;40.27,-37.9,;41.62,-37.16,;42.94,-37.97,;42.9,-39.51,;41.54,-40.24,;40.23,-39.43,;38.87,-40.17,;37.56,-39.36,;38.83,-41.71,;37.48,-42.44,;37.43,-43.97,;38.74,-44.78,;38.69,-46.31,;39.99,-47.13,;41.35,-46.4,;41.41,-44.86,;40.1,-44.05,;40.15,-42.51,;44.21,-40.32,;45.57,-39.59,;44.17,-41.86,;45.48,-42.66,;46.27,-41.34,;47.02,-42.69,;45.43,-44.21,;46.75,-45.01,;46.72,-46.55,;48.03,-47.35,;45.37,-47.29,;46.7,-48.08,)| Show InChI InChI=1S/C36H44N4O5SSi/c1-5-6-16-33-34(32(25-41)37-40(33)29-14-8-7-9-15-29)30-18-17-27(35(42)38-46(44,45)21-22-47(2,3)4)23-31(30)36(43)39-20-19-26-12-10-11-13-28(26)24-39/h7-15,17-18,23,41H,5-6,16,19-22,24-25H2,1-4H3,(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50020919
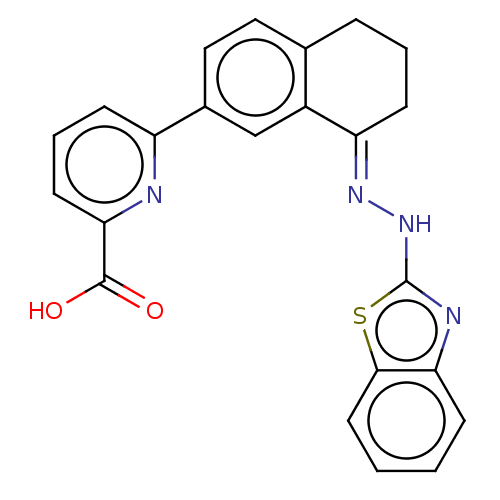
(CHEMBL3287282)Show SMILES OC(=O)c1cccc(n1)-c1ccc2CCC\C(=N/Nc3nc4ccccc4s3)c2c1 Show InChI InChI=1S/C23H18N4O2S/c28-22(29)20-9-4-7-17(24-20)15-12-11-14-5-3-8-18(16(14)13-15)26-27-23-25-19-6-1-2-10-21(19)30-23/h1-2,4,6-7,9-13H,3,5,8H2,(H,25,27)(H,28,29)/b26-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066764
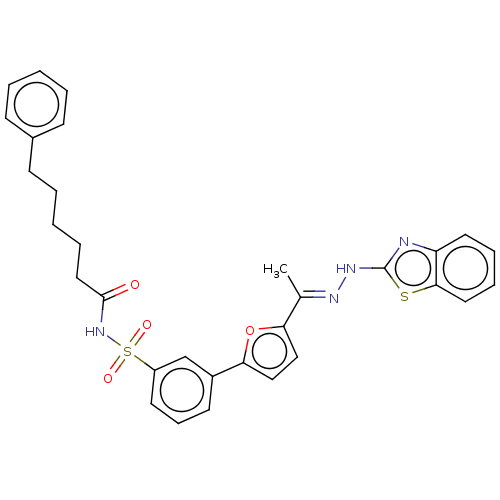
(CHEMBL3400496)Show SMILES C\C(=N/Nc1nc2ccccc2s1)c1ccc(o1)-c1cccc(c1)S(=O)(=O)NC(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C31H30N4O4S2/c1-22(33-34-31-32-26-16-8-9-17-29(26)40-31)27-19-20-28(39-27)24-14-10-15-25(21-24)41(37,38)35-30(36)18-7-3-6-13-23-11-4-2-5-12-23/h2,4-5,8-12,14-17,19-21H,3,6-7,13,18H2,1H3,(H,32,34)(H,35,36)/b33-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50066762
(CHEMBL3125476)Show SMILES CCCN(C(=O)N[C@@H](CSCc1ccc(Br)cc1)C(O)=O)C(=O)c1cccc(c1)C#Cc1ccc(F)cc1F |r| Show InChI InChI=1S/C29H25BrF2N2O4S/c1-2-14-34(29(38)33-26(28(36)37)18-39-17-20-7-11-23(30)12-8-20)27(35)22-5-3-4-19(15-22)6-9-21-10-13-24(31)16-25(21)32/h3-5,7-8,10-13,15-16,26H,2,14,17-18H2,1H3,(H,33,38)(H,36,37)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50448930
(CHEMBL3125475)Show SMILES CCCN(C(=O)N[C@@H](CSCC(C)C)C(O)=O)C(=O)c1cccc(c1)C#Cc1ccc(F)cc1F |r| Show InChI InChI=1S/C26H28F2N2O4S/c1-4-12-30(26(34)29-23(25(32)33)16-35-15-17(2)3)24(31)20-7-5-6-18(13-20)8-9-19-10-11-21(27)14-22(19)28/h5-7,10-11,13-14,17,23H,4,12,15-16H2,1-3H3,(H,29,34)(H,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153811

(14-cyclopentyl-7-ethyl-7-hydroxy-(7S)-7,8,11,13-te...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(C4CCCC4)c3Cn1c2=O Show InChI InChI=1S/C26H24N2O6/c1-2-26(31)17-8-19-23-15(10-28(19)24(29)16(17)11-32-25(26)30)22(13-5-3-4-6-13)14-7-20-21(34-12-33-20)9-18(14)27-23/h7-9,13,31H,2-6,10-12H2,1H3/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153819
((S)-11-Butyl-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-o...)Show SMILES CCCCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2ccccc12 Show InChI InChI=1S/C24H24N2O4/c1-3-5-8-14-15-9-6-7-10-19(15)25-21-16(14)12-26-20(21)11-18-17(22(26)27)13-30-23(28)24(18,29)4-2/h6-7,9-11,29H,3-5,8,12-13H2,1-2H3/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50036133
(7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4cc3Cn1c2=O Show InChI InChI=1S/C21H16N2O6/c1-2-21(26)13-5-15-18-11(7-23(15)19(24)12(13)8-27-20(21)25)3-10-4-16-17(29-9-28-16)6-14(10)22-18/h3-6,26H,2,7-9H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153813

(7-ethyl-14-(4-fluorophenyl)-7-hydroxy-(7S)-7,8,11,...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(c3Cn1c2=O)-c1ccc(F)cc1 Show InChI InChI=1S/C27H19FN2O6/c1-2-27(33)18-8-20-24-16(10-30(20)25(31)17(18)11-34-26(27)32)23(13-3-5-14(28)6-4-13)15-7-21-22(36-12-35-21)9-19(15)29-24/h3-9,33H,2,10-12H2,1H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153809
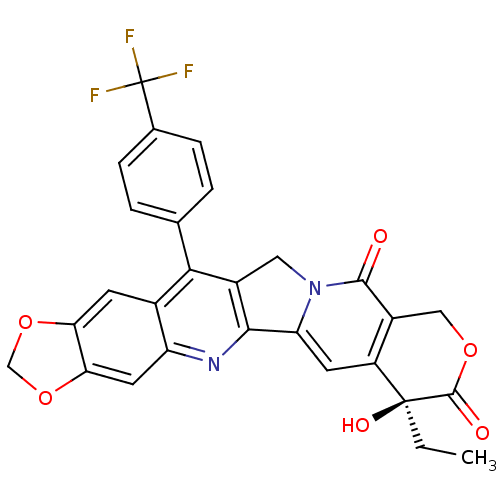
(7-ethyl-7-hydroxy-14-(4-trifluoromethylphenyl)-(7S...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(c3Cn1c2=O)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H19F3N2O6/c1-2-27(36)18-8-20-24-16(10-33(20)25(34)17(18)11-37-26(27)35)23(13-3-5-14(6-4-13)28(29,30)31)15-7-21-22(39-12-38-21)9-19(15)32-24/h3-9,36H,2,10-12H2,1H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50092821
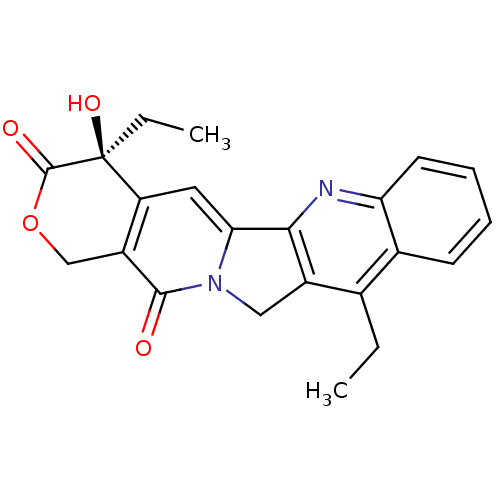
((R)-4-Ethyl-4-methyl-1,4a,12,13a-tetrahydro-4H-2-o...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2ccccc12 Show InChI InChI=1S/C22H20N2O4/c1-3-12-13-7-5-6-8-17(13)23-19-14(12)10-24-18(19)9-16-15(20(24)25)11-28-21(26)22(16,27)4-2/h5-9,27H,3-4,10-11H2,1-2H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153816
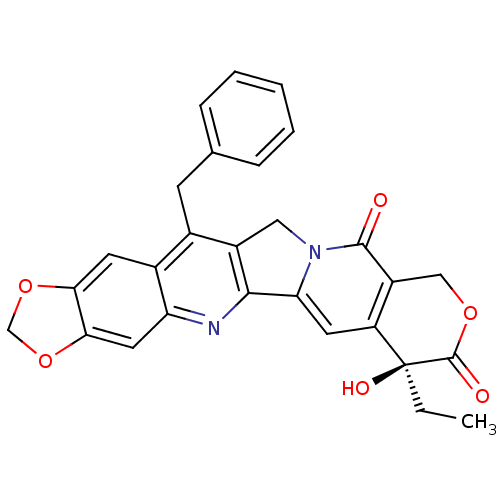
(14-benzyl-7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahy...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(Cc4ccccc4)c3Cn1c2=O Show InChI InChI=1S/C28H22N2O6/c1-2-28(33)20-10-22-25-18(12-30(22)26(31)19(20)13-34-27(28)32)16(8-15-6-4-3-5-7-15)17-9-23-24(36-14-35-23)11-21(17)29-25/h3-7,9-11,33H,2,8,12-14H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153807
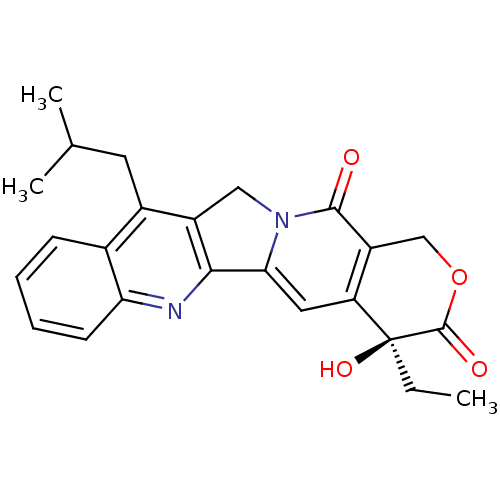
((S)-4-Ethyl-4-hydroxy-11-isobutyl-1,12-dihydro-4H-...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccccc4c(CC(C)C)c3Cn1c2=O Show InChI InChI=1S/C24H24N2O4/c1-4-24(29)18-10-20-21-16(11-26(20)22(27)17(18)12-30-23(24)28)15(9-13(2)3)14-7-5-6-8-19(14)25-21/h5-8,10,13,29H,4,9,11-12H2,1-3H3/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153814

(7-ethyl-7-hydroxy-14-(4-methylphenyl)-(7S)-7,8,11,...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(c3Cn1c2=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H19ClN2O6/c1-2-27(33)18-8-20-24-16(10-30(20)25(31)17(18)11-34-26(27)32)23(13-3-5-14(28)6-4-13)15-7-21-22(36-12-35-21)9-19(15)29-24/h3-9,33H,2,10-12H2,1H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153818
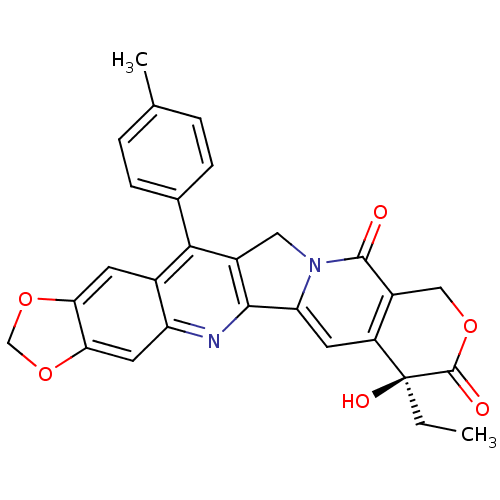
(7-ethyl-7-hydroxy-14-(4-methylphenyl)-(7S)-7,8,11,...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(c3Cn1c2=O)-c1ccc(C)cc1 Show InChI InChI=1S/C28H22N2O6/c1-3-28(33)19-9-21-25-17(11-30(21)26(31)18(19)12-34-27(28)32)24(15-6-4-14(2)5-7-15)16-8-22-23(36-13-35-22)10-20(16)29-25/h4-10,33H,3,11-13H2,1-2H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153817
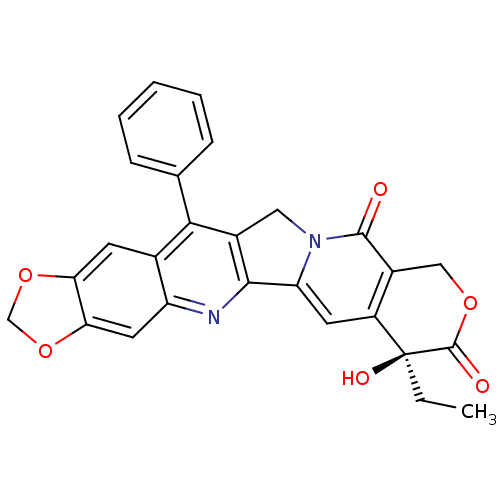
(7-ethyl-7-hydroxy-14-phenyl-(7S)-7,8,11,13-tetrahy...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(c3Cn1c2=O)-c1ccccc1 Show InChI InChI=1S/C27H20N2O6/c1-2-27(32)18-9-20-24-16(11-29(20)25(30)17(18)12-33-26(27)31)23(14-6-4-3-5-7-14)15-8-21-22(35-13-34-21)10-19(15)28-24/h3-10,32H,2,11-13H2,1H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153808
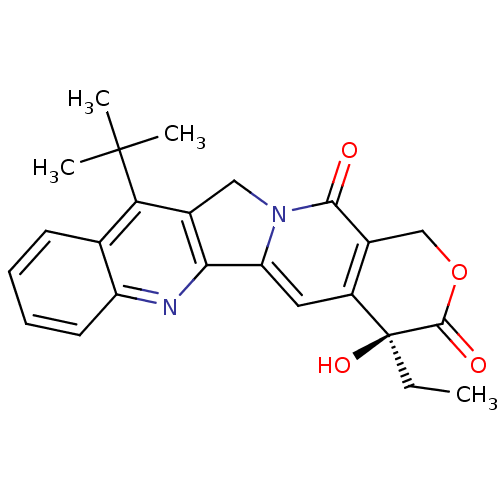
((S)-11-tert-Butyl-4-ethyl-4-hydroxy-1,12-dihydro-4...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccccc4c(c3Cn1c2=O)C(C)(C)C Show InChI InChI=1S/C24H24N2O4/c1-5-24(29)16-10-18-20-14(11-26(18)21(27)15(16)12-30-22(24)28)19(23(2,3)4)13-8-6-7-9-17(13)25-20/h6-10,29H,5,11-12H2,1-4H3/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153815

(14-cyclohexyl-7-ethyl-7-hydroxy-(7S)-7,8,11,13-tet...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4c(C4CCCCC4)c3Cn1c2=O Show InChI InChI=1S/C27H26N2O6/c1-2-27(32)18-9-20-24-16(11-29(20)25(30)17(18)12-33-26(27)31)23(14-6-4-3-5-7-14)15-8-21-22(35-13-34-21)10-19(15)28-24/h8-10,14,32H,2-7,11-13H2,1H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50008923

((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccccc4cc3Cn1c2=O |r| Show InChI InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153812
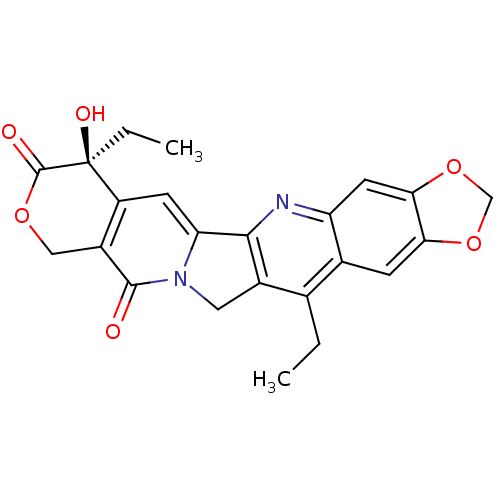
(7,14-diethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-1...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2cc3OCOc3cc12 Show InChI InChI=1S/C23H20N2O6/c1-3-11-12-5-18-19(31-10-30-18)7-16(12)24-20-13(11)8-25-17(20)6-15-14(21(25)26)9-29-22(27)23(15,28)4-2/h5-7,28H,3-4,8-10H2,1-2H3/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50153810
((S)-11-Benzyl-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccccc4c(Cc4ccccc4)c3Cn1c2=O Show InChI InChI=1S/C27H22N2O4/c1-2-27(32)21-13-23-24-19(14-29(23)25(30)20(21)15-33-26(27)31)18(12-16-8-4-3-5-9-16)17-10-6-7-11-22(17)28-24/h3-11,13,32H,2,12,14-15H2,1H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Effective concentration against DNA topoisomerase I |
Bioorg Med Chem Lett 14: 5377-81 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.010
BindingDB Entry DOI: 10.7270/Q2D799WJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50060993
(4'-FLUORO-1,1'-BIPHENYL-4-CARBOXYLIC ACID | 4'-Flu...)Show InChI InChI=1S/C13H9FO2/c14-12-7-5-10(6-8-12)9-1-3-11(4-2-9)13(15)16/h1-8H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50270875

(5,6,7,8-tetrahydro-1-naphthol | CHEMBL449132)Show InChI InChI=1S/C10H12O/c11-10-7-3-5-8-4-1-2-6-9(8)10/h3,5,7,11H,1-2,4,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 4.30E+6 | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Bcl-xL (unknown origin) measured after 1 hr incubation by fluorescence polarization assay |
Bioorg Med Chem 23: 1747-57 (2015)
Article DOI: 10.1016/j.bmc.2015.02.060
BindingDB Entry DOI: 10.7270/Q2F191D6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data