 Found 47 hits with Last Name = 'vyas' and Initial = 'vk'
Found 47 hits with Last Name = 'vyas' and Initial = 'vk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max (Soybean)) | BDBM192705
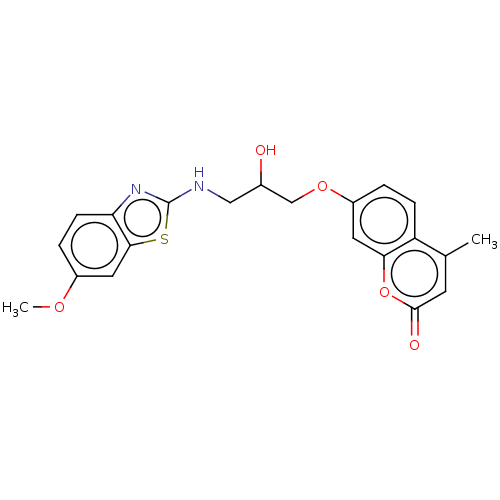
(7-(2-hydroxy-3-(6-methoxybenzo[d]thiazol-2-ylamino...)Show SMILES COc1ccc2nc(NCC(O)COc3ccc4c(C)cc(=O)oc4c3)sc2c1 Show InChI InChI=1S/C21H20N2O5S/c1-12-7-20(25)28-18-8-15(3-5-16(12)18)27-11-13(24)10-22-21-23-17-6-4-14(26-2)9-19(17)29-21/h3-9,13,24H,10-11H2,1-2H3,(H,22,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12 | n/a | n/a | n/a | n/a | 9.0 | 25 |
Nirma University
| Assay Description
It is determined by kinetic mode of spectro-photometric determination method,which was performed by recording the rate of change in absorbance at 234... |
Bioorg Chem 67: 130-8 (2016)
Article DOI: 10.1016/j.bioorg.2016.06.004
BindingDB Entry DOI: 10.7270/Q2M907GG |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max (Soybean)) | BDBM192703
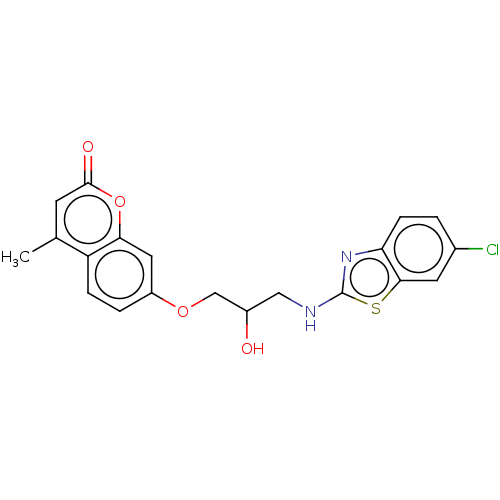
(7-(3-(6-chlorobenzo[d]thiazol-2-ylamino)-2-hydroxy...)Show SMILES Cc1cc(=O)oc2cc(OCC(O)CNc3nc4ccc(Cl)cc4s3)ccc12 Show InChI InChI=1S/C20H17ClN2O4S/c1-11-6-19(25)27-17-8-14(3-4-15(11)17)26-10-13(24)9-22-20-23-16-5-2-12(21)7-18(16)28-20/h2-8,13,24H,9-10H2,1H3,(H,22,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79 | n/a | n/a | n/a | n/a | 9.0 | 25 |
Nirma University
| Assay Description
It is determined by kinetic mode of spectro-photometric determination method,which was performed by recording the rate of change in absorbance at 234... |
Bioorg Chem 67: 130-8 (2016)
Article DOI: 10.1016/j.bioorg.2016.06.004
BindingDB Entry DOI: 10.7270/Q2M907GG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max (Soybean)) | BDBM192701
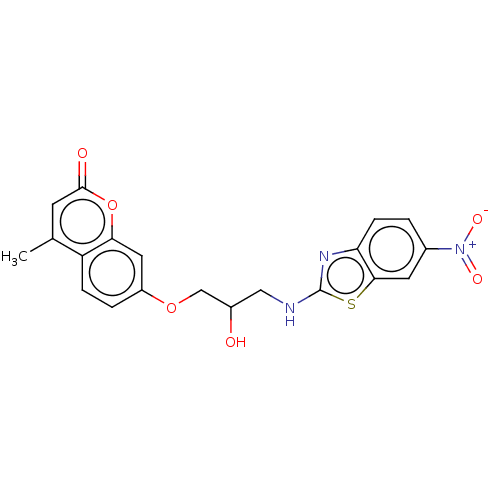
(7-(2-hydroxy-3-(6-nitrobenzo[d]thiazol-2-ylamino)p...)Show SMILES Cc1cc(=O)oc2cc(OCC(O)CNc3nc4ccc(cc4s3)[N+]([O-])=O)ccc12 Show InChI InChI=1S/C20H17N3O6S/c1-11-6-19(25)29-17-8-14(3-4-15(11)17)28-10-13(24)9-21-20-22-16-5-2-12(23(26)27)7-18(16)30-20/h2-8,13,24H,9-10H2,1H3,(H,21,22) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.24 | n/a | n/a | n/a | n/a | 9.0 | 25 |
Nirma University
| Assay Description
It is determined by kinetic mode of spectro-photometric determination method,which was performed by recording the rate of change in absorbance at 234... |
Bioorg Chem 67: 130-8 (2016)
Article DOI: 10.1016/j.bioorg.2016.06.004
BindingDB Entry DOI: 10.7270/Q2M907GG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max (Soybean)) | BDBM192702
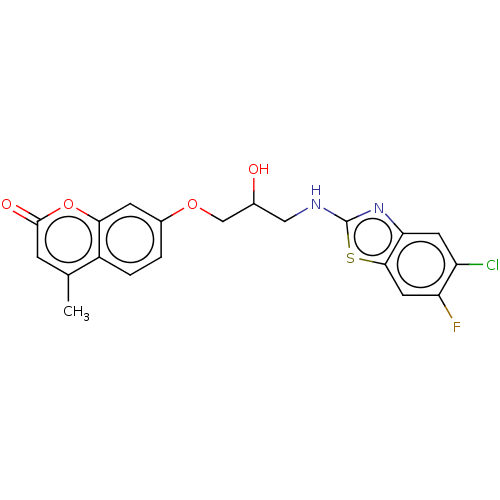
(7-(3-(5-chloro-6-fluorobenzo[d]thiazol-2-ylamino)-...)Show SMILES Cc1cc(=O)oc2cc(OCC(O)CNc3nc4cc(Cl)c(F)cc4s3)ccc12 Show InChI InChI=1S/C20H16ClFN2O4S/c1-10-4-19(26)28-17-5-12(2-3-13(10)17)27-9-11(25)8-23-20-24-16-6-14(21)15(22)7-18(16)29-20/h2-7,11,25H,8-9H2,1H3,(H,23,24) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.16 | n/a | n/a | n/a | n/a | 9.0 | 25 |
Nirma University
| Assay Description
It is determined by kinetic mode of spectro-photometric determination method,which was performed by recording the rate of change in absorbance at 234... |
Bioorg Chem 67: 130-8 (2016)
Article DOI: 10.1016/j.bioorg.2016.06.004
BindingDB Entry DOI: 10.7270/Q2M907GG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM592358
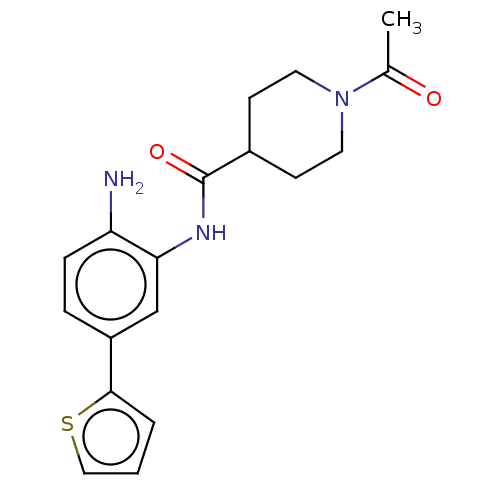
(US11572368, Compound 35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50069959

(Brequinar sodium | CHEMBL300058 | Sodium; 6-fluoro...)Show SMILES Cc1c(nc2ccc(F)cc2c1C([O-])=O)-c1ccc(cc1)-c1ccccc1F Show InChI InChI=1S/C23H15F2NO2/c1-13-21(23(27)28)18-12-16(24)10-11-20(18)26-22(13)15-8-6-14(7-9-15)17-4-2-3-5-19(17)25/h2-12H,1H3,(H,27,28)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Glycine max (Soybean)) | BDBM192704

(7-(3-(5,6-dichlorobenzo[d]thiazol-2-ylamino)-2-hyd...)Show SMILES Cc1cc(=O)oc2cc(OCC(O)CNc3nc4cc(Cl)c(Cl)cc4s3)ccc12 Show InChI InChI=1S/C20H16Cl2N2O4S/c1-10-4-19(26)28-17-5-12(2-3-13(10)17)27-9-11(25)8-23-20-24-16-6-14(21)15(22)7-18(16)29-20/h2-7,11,25H,8-9H2,1H3,(H,23,24) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | 9.0 | 25 |
Nirma University
| Assay Description
It is determined by kinetic mode of spectro-photometric determination method,which was performed by recording the rate of change in absorbance at 234... |
Bioorg Chem 67: 130-8 (2016)
Article DOI: 10.1016/j.bioorg.2016.06.004
BindingDB Entry DOI: 10.7270/Q2M907GG |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50069959

(Brequinar sodium | CHEMBL300058 | Sodium; 6-fluoro...)Show SMILES Cc1c(nc2ccc(F)cc2c1C([O-])=O)-c1ccc(cc1)-c1ccccc1F Show InChI InChI=1S/C23H15F2NO2/c1-13-21(23(27)28)18-12-16(24)10-11-20(18)26-22(13)15-8-6-14(7-9-15)17-4-2-3-5-19(17)25/h2-12H,1H3,(H,27,28)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM592358

(US11572368, Compound 35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50470579
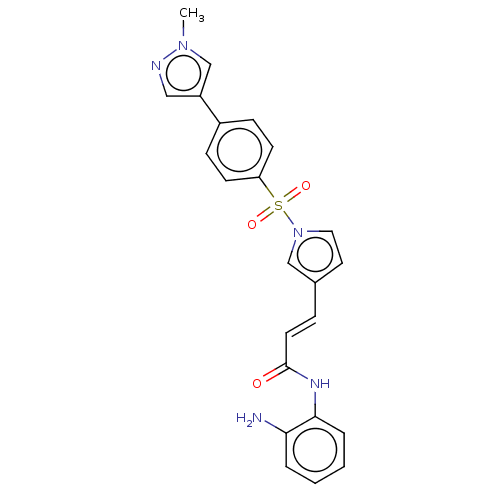
(4sc-202 | Domatinostat)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C23H21N5O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24/h2-16H,24H2,1H3,(H,26,29)/b11-6+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020687
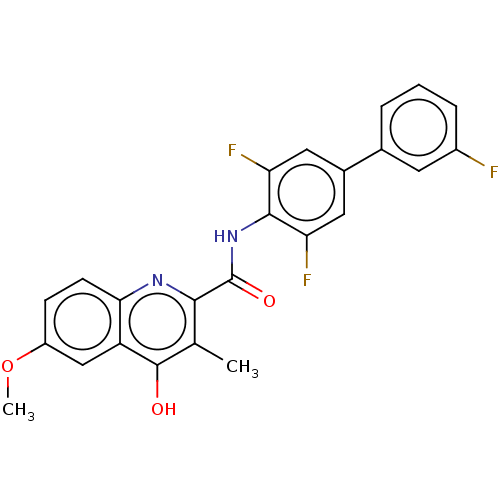
(CHEMBL3290836)Show SMILES COc1ccc2nc(C(=O)Nc3c(F)cc(cc3F)-c3cccc(F)c3)c(C)c(O)c2c1 Show InChI InChI=1S/C24H17F3N2O3/c1-12-21(28-20-7-6-16(32-2)11-17(20)23(12)30)24(31)29-22-18(26)9-14(10-19(22)27)13-4-3-5-15(25)8-13/h3-11H,1-2H3,(H,28,30)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020688
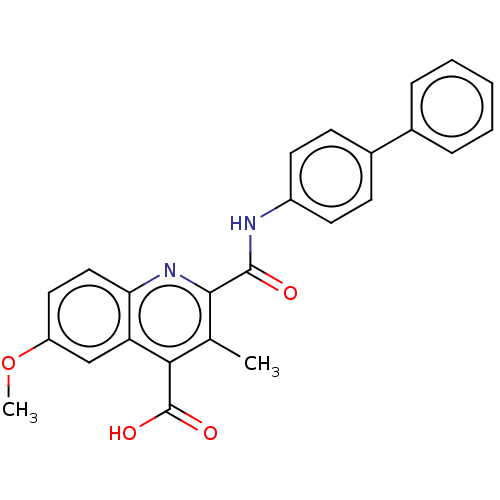
(CHEMBL3290847)Show SMILES COc1ccc2nc(C(=O)Nc3ccc(cc3)-c3ccccc3)c(C)c(C(O)=O)c2c1 Show InChI InChI=1S/C25H20N2O4/c1-15-22(25(29)30)20-14-19(31-2)12-13-21(20)27-23(15)24(28)26-18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-14H,1-2H3,(H,26,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50470579

(4sc-202 | Domatinostat)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C23H21N5O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24/h2-16H,24H2,1H3,(H,26,29)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50470579

(4sc-202 | Domatinostat)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C23H21N5O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24/h2-16H,24H2,1H3,(H,26,29)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532171
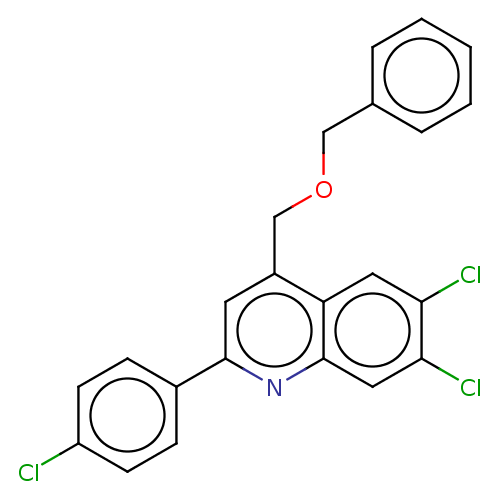
(CHEMBL4543612)Show SMILES Clc1ccc(cc1)-c1cc(COCc2ccccc2)c2cc(Cl)c(Cl)cc2n1 Show InChI InChI=1S/C23H16Cl3NO/c24-18-8-6-16(7-9-18)22-10-17(14-28-13-15-4-2-1-3-5-15)19-11-20(25)21(26)12-23(19)27-22/h1-12H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020689
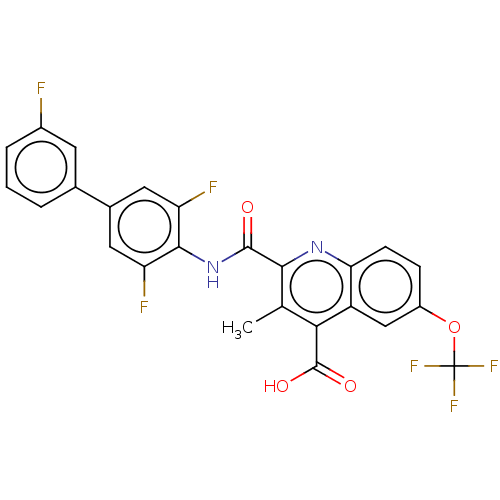
(CHEMBL3290845)Show SMILES Cc1c(nc2ccc(OC(F)(F)F)cc2c1C(O)=O)C(=O)Nc1c(F)cc(cc1F)-c1cccc(F)c1 Show InChI InChI=1S/C25H14F6N2O4/c1-11-20(24(35)36)16-10-15(37-25(29,30)31)5-6-19(16)32-21(11)23(34)33-22-17(27)8-13(9-18(22)28)12-3-2-4-14(26)7-12/h2-10H,1H3,(H,33,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532167
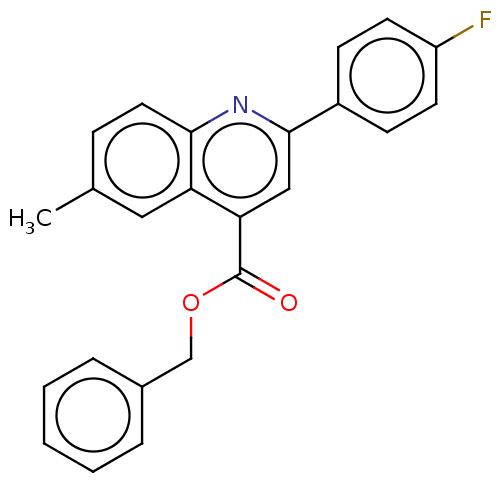
(CHEMBL4460961)Show SMILES Cc1ccc2nc(cc(C(=O)OCc3ccccc3)c2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C24H18FNO2/c1-16-7-12-22-20(13-16)21(24(27)28-15-17-5-3-2-4-6-17)14-23(26-22)18-8-10-19(25)11-9-18/h2-14H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532170
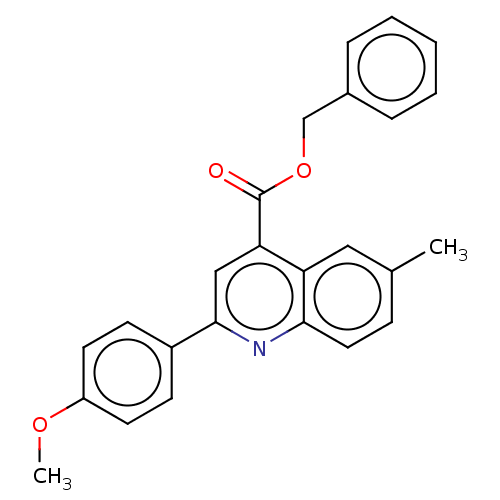
(CHEMBL4464999)Show SMILES COc1ccc(cc1)-c1cc(C(=O)OCc2ccccc2)c2cc(C)ccc2n1 Show InChI InChI=1S/C25H21NO3/c1-17-8-13-23-21(14-17)22(25(27)29-16-18-6-4-3-5-7-18)15-24(26-23)19-9-11-20(28-2)12-10-19/h3-15H,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020694
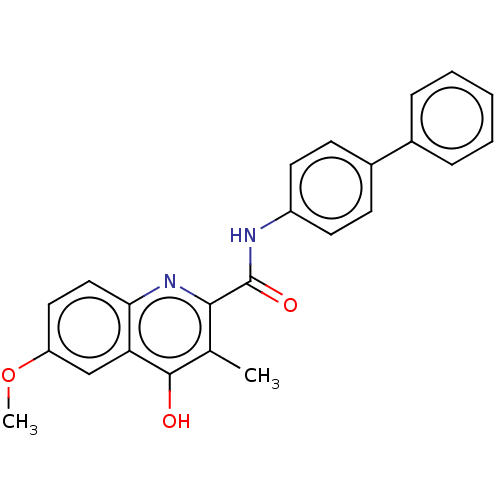
(CHEMBL3286441)Show SMILES COc1ccc2nc(C(=O)Nc3ccc(cc3)-c3ccccc3)c(C)c(O)c2c1 Show InChI InChI=1S/C24H20N2O3/c1-15-22(26-21-13-12-19(29-2)14-20(21)23(15)27)24(28)25-18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-14H,1-2H3,(H,25,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
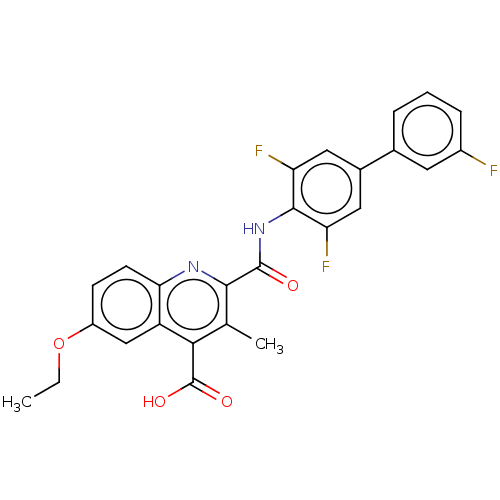
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020695
(CHEMBL3290843)Show SMILES CCOc1ccc2nc(C(=O)Nc3c(F)cc(cc3F)-c3cccc(F)c3)c(C)c(C(O)=O)c2c1 Show InChI InChI=1S/C26H19F3N2O4/c1-3-35-17-7-8-21-18(12-17)22(26(33)34)13(2)23(30-21)25(32)31-24-19(28)10-15(11-20(24)29)14-5-4-6-16(27)9-14/h4-12H,3H2,1-2H3,(H,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
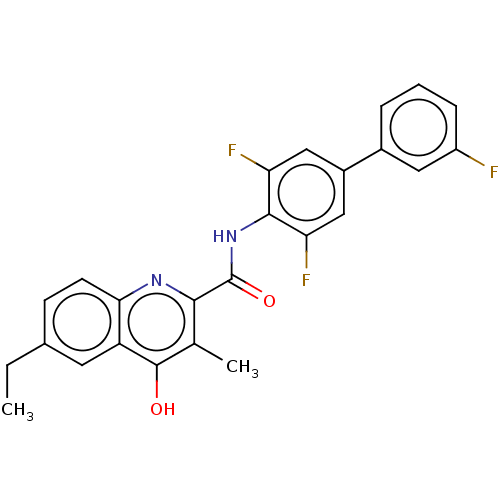
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020690
(CHEMBL3290833)Show SMILES CCc1ccc2nc(C(=O)Nc3c(F)cc(cc3F)-c3cccc(F)c3)c(C)c(O)c2c1 Show InChI InChI=1S/C25H19F3N2O2/c1-3-14-7-8-21-18(9-14)24(31)13(2)22(29-21)25(32)30-23-19(27)11-16(12-20(23)28)15-5-4-6-17(26)10-15/h4-12H,3H2,1-2H3,(H,29,31)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532165
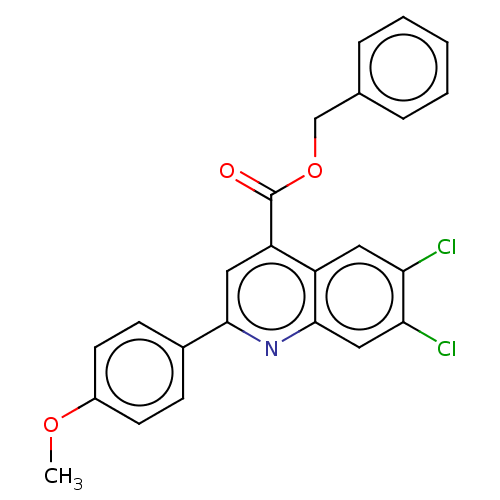
(CHEMBL4579382)Show SMILES COc1ccc(cc1)-c1cc(C(=O)OCc2ccccc2)c2cc(Cl)c(Cl)cc2n1 Show InChI InChI=1S/C24H17Cl2NO3/c1-29-17-9-7-16(8-10-17)22-12-19(18-11-20(25)21(26)13-23(18)27-22)24(28)30-14-15-5-3-2-4-6-15/h2-13H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020691
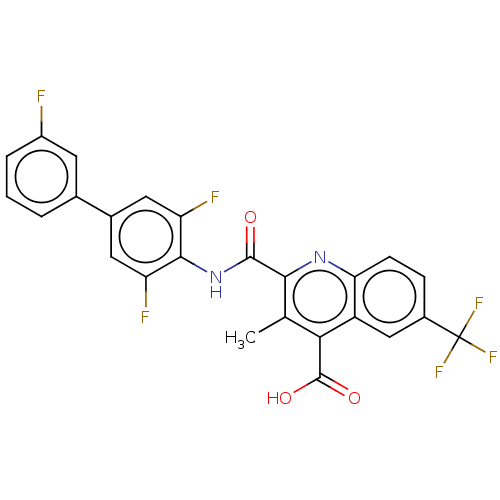
(CHEMBL3290846)Show SMILES Cc1c(nc2ccc(cc2c1C(O)=O)C(F)(F)F)C(=O)Nc1c(F)cc(cc1F)-c1cccc(F)c1 Show InChI InChI=1S/C25H14F6N2O3/c1-11-20(24(35)36)16-10-14(25(29,30)31)5-6-19(16)32-21(11)23(34)33-22-17(27)8-13(9-18(22)28)12-3-2-4-15(26)7-12/h2-10H,1H3,(H,33,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020692

(CHEMBL3290842)Show SMILES Cc1c(nc2ccc(F)cc2c1C(O)=O)C(=O)Nc1c(F)cc(cc1F)-c1cccc(F)c1 Show InChI InChI=1S/C24H14F4N2O3/c1-11-20(24(32)33)16-10-15(26)5-6-19(16)29-21(11)23(31)30-22-17(27)8-13(9-18(22)28)12-3-2-4-14(25)7-12/h2-10H,1H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50020693
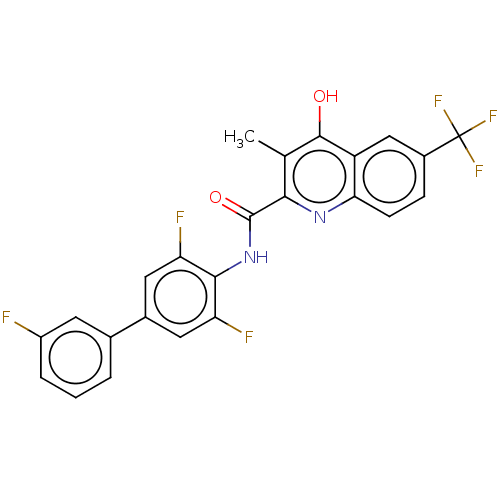
(CHEMBL3290838)Show SMILES Cc1c(O)c2cc(ccc2nc1C(=O)Nc1c(F)cc(cc1F)-c1cccc(F)c1)C(F)(F)F Show InChI InChI=1S/C24H14F6N2O2/c1-11-20(31-19-6-5-14(24(28,29)30)10-16(19)22(11)33)23(34)32-21-17(26)8-13(9-18(21)27)12-3-2-4-15(25)7-12/h2-10H,1H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHODH activity after 20 mins by DCIP dye based colorimetric analysis in presence of 1 mM L-dihydroorotate and 1 mM Cq... |
Eur J Med Chem 82: 385-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.064
BindingDB Entry DOI: 10.7270/Q20V8FB4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532166
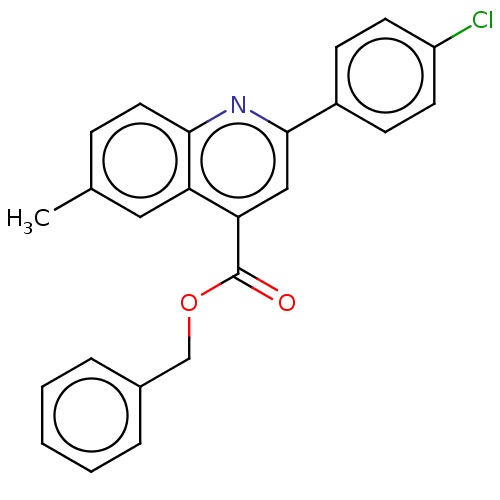
(CHEMBL4586996)Show SMILES Cc1ccc2nc(cc(C(=O)OCc3ccccc3)c2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H18ClNO2/c1-16-7-12-22-20(13-16)21(24(27)28-15-17-5-3-2-4-6-17)14-23(26-22)18-8-10-19(25)11-9-18/h2-14H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532168
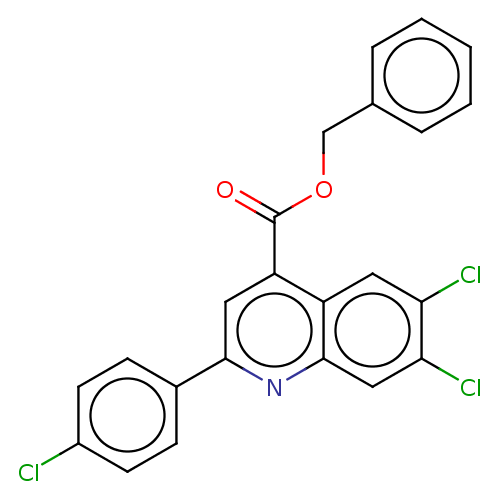
(CHEMBL4437585)Show SMILES Clc1ccc(cc1)-c1cc(C(=O)OCc2ccccc2)c2cc(Cl)c(Cl)cc2n1 Show InChI InChI=1S/C23H14Cl3NO2/c24-16-8-6-15(7-9-16)21-11-18(17-10-19(25)20(26)12-22(17)27-21)23(28)29-13-14-4-2-1-3-5-14/h1-12H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50470579

(4sc-202 | Domatinostat)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C23H21N5O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24/h2-16H,24H2,1H3,(H,26,29)/b11-6+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532172
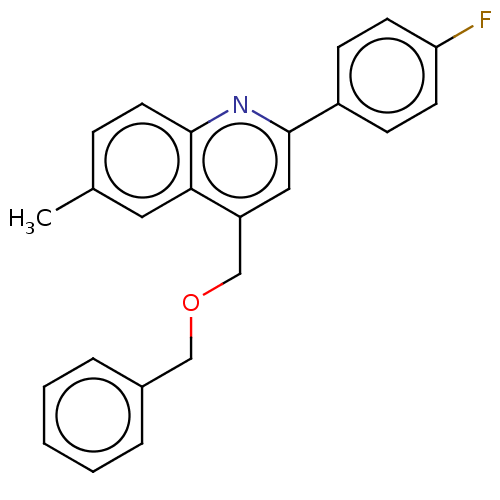
(CHEMBL4544880)Show InChI InChI=1S/C24H20FNO/c1-17-7-12-23-22(13-17)20(16-27-15-18-5-3-2-4-6-18)14-24(26-23)19-8-10-21(25)11-9-19/h2-14H,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50532169
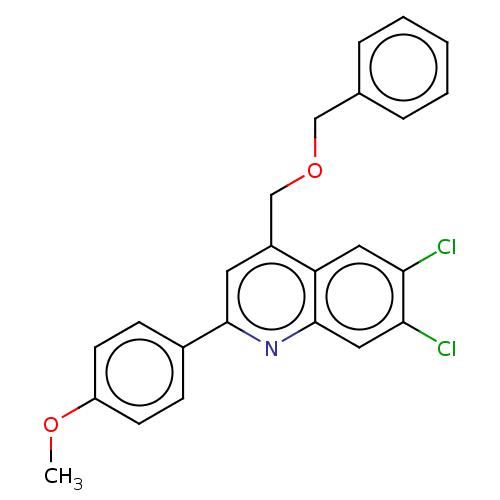
(CHEMBL4460949)Show SMILES COc1ccc(cc1)-c1cc(COCc2ccccc2)c2cc(Cl)c(Cl)cc2n1 Show InChI InChI=1S/C24H19Cl2NO2/c1-28-19-9-7-17(8-10-19)23-11-18(15-29-14-16-5-3-2-4-6-16)20-12-21(25)22(26)13-24(20)27-23/h2-13H,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nirma University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using DHO as substrate measured after ... |
Bioorg Med Chem Lett 29: 917-922 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.038
BindingDB Entry DOI: 10.7270/Q29Z98CQ |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50470579

(4sc-202 | Domatinostat)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C23H21N5O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24/h2-16H,24H2,1H3,(H,26,29)/b11-6+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50470579

(4sc-202 | Domatinostat)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C23H21N5O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24/h2-16H,24H2,1H3,(H,26,29)/b11-6+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data