 Found 169 hits with Last Name = 'wagner' and Initial = 'b'
Found 169 hits with Last Name = 'wagner' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | -50.4 | 13 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM178095
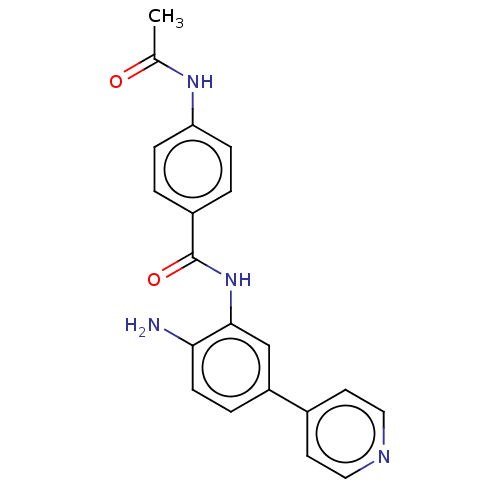
(BRD2492)Show SMILES CC(=O)Nc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1ccncc1 Show InChI InChI=1S/C20H18N4O2/c1-13(25)23-17-5-2-15(3-6-17)20(26)24-19-12-16(4-7-18(19)21)14-8-10-22-11-9-14/h2-12H,21H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -48.6 | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM178095

(BRD2492)Show SMILES CC(=O)Nc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1ccncc1 Show InChI InChI=1S/C20H18N4O2/c1-13(25)23-17-5-2-15(3-6-17)20(26)24-19-12-16(4-7-18(19)21)14-8-10-22-11-9-14/h2-12H,21H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | -44.8 | 19 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | -43.4 | 46 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511169

(CHEMBL4551377)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(N)nc(N)c1 |r| Show InChI InChI=1S/C20H26N6O2.2C2HF3O2/c21-15(9-13-5-2-1-3-6-13)20(28)26-8-4-7-16(26)19(27)24-12-14-10-17(22)25-18(23)11-14;2*3-2(4,5)1(6)7/h1-3,5-6,10-11,15-16H,4,7-9,12,21H2,(H,24,27)(H4,22,23,25);2*(H,6,7)/t15-,16+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM178100
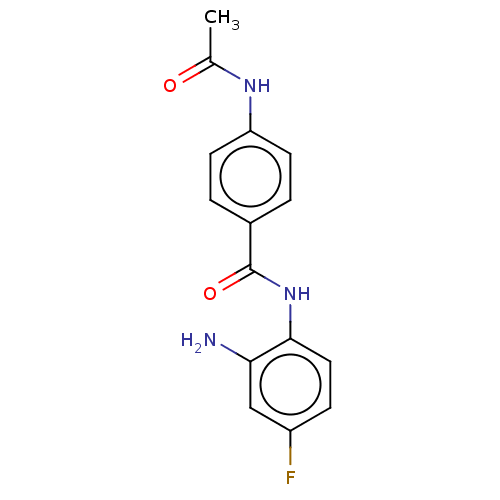
(BRD3308 | US11377423, Cmpd 1)Show InChI InChI=1S/C15H14FN3O2/c1-9(20)18-12-5-2-10(3-6-12)15(21)19-14-7-4-11(16)8-13(14)17/h2-8H,17H2,1H3,(H,18,20)(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | -43.0 | 64 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | -42.4 | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511166

(CHEMBL4513436)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(N)nc1 |r| Show InChI InChI=1S/C20H25N5O2.2C2HF3O2/c21-16(11-14-5-2-1-3-6-14)20(27)25-10-4-7-17(25)19(26)24-13-15-8-9-18(22)23-12-15;2*3-2(4,5)1(6)7/h1-3,5-6,8-9,12,16-17H,4,7,10-11,13,21H2,(H2,22,23)(H,24,26);2*(H,6,7)/t16-,17+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 223 | -38.0 | 147 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511167

(CHEMBL4563187)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccnc(N)c1 |r| Show InChI InChI=1S/C20H25N5O2.2C2HF3O2/c21-16(11-14-5-2-1-3-6-14)20(27)25-10-4-7-17(25)19(26)24-13-15-8-9-23-18(22)12-15;2*3-2(4,5)1(6)7/h1-3,5-6,8-9,12,16-17H,4,7,10-11,13,21H2,(H2,22,23)(H,24,26);2*(H,6,7)/t16-,17+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 500 | -36.0 | 398 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511168

(CHEMBL4465587)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccncc1 |r| Show InChI InChI=1S/C26H28N4O2.2C2HF3O2/c27-24(23(20-8-3-1-4-9-20)21-10-5-2-6-11-21)26(32)30-17-7-12-22(30)25(31)29-18-19-13-15-28-16-14-19;2*3-2(4,5)1(6)7/h1-6,8-11,13-16,22-24H,7,12,17-18,27H2,(H,29,31);2*(H,6,7)/t22-,24+;;/m0../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM178100

(BRD3308 | US11377423, Cmpd 1)Show InChI InChI=1S/C15H14FN3O2/c1-9(20)18-12-5-2-10(3-6-12)15(21)19-14-7-4-11(16)8-13(14)17/h2-8H,17H2,1H3,(H,18,20)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | -30.2 | 1.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM178100

(BRD3308 | US11377423, Cmpd 1)Show InChI InChI=1S/C15H14FN3O2/c1-9(20)18-12-5-2-10(3-6-12)15(21)19-14-7-4-11(16)8-13(14)17/h2-8H,17H2,1H3,(H,18,20)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | -29.7 | 1.15E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM178095

(BRD2492)Show SMILES CC(=O)Nc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1ccncc1 Show InChI InChI=1S/C20H18N4O2/c1-13(25)23-17-5-2-15(3-6-17)20(26)24-19-12-16(4-7-18(19)21)14-8-10-22-11-9-14/h2-12H,21H2,1H3,(H,23,25)(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | -27.7 | 2.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM559897
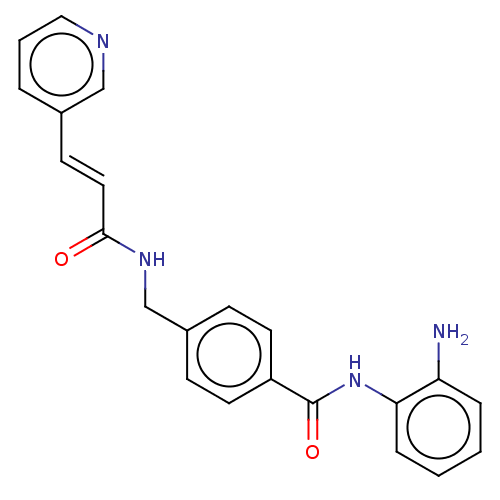
(US11377423, Cmpd 3A)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cccnc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24624
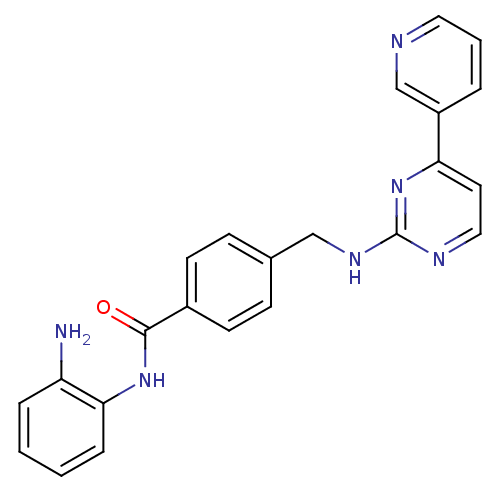
(CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50457079
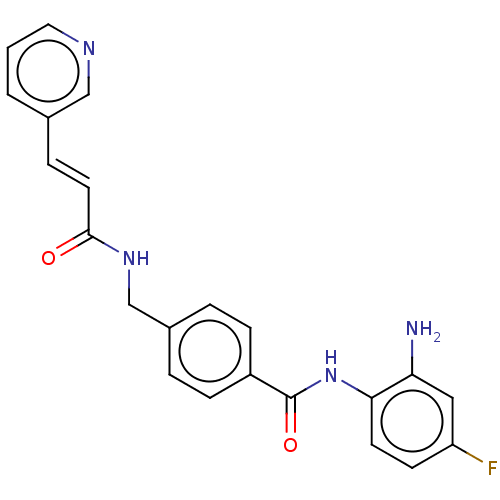
(CS-055 | CS055 | Chidamide | Epidaza in China | HB...)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cccnc2)cc1 Show InChI InChI=1S/C22H19FN4O2/c23-18-8-9-20(19(24)12-18)27-22(29)17-6-3-16(4-7-17)14-26-21(28)10-5-15-2-1-11-25-13-15/h1-13H,14,24H2,(H,26,28)(H,27,29)/b10-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM559896
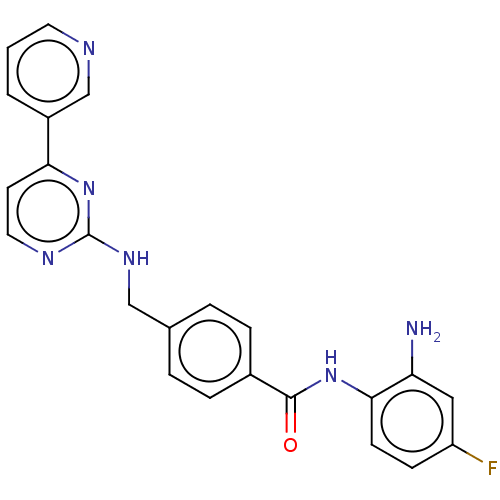
(US11377423, Cmpd 2A)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410
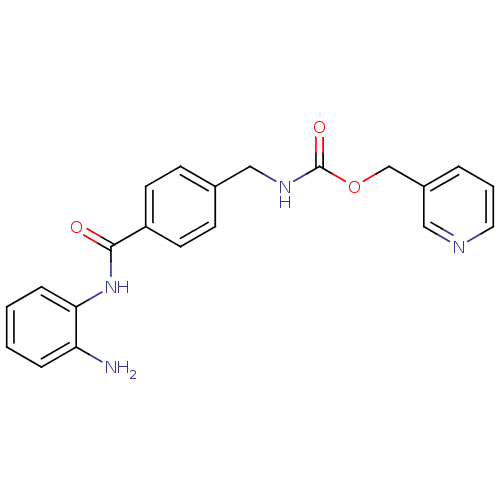
(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM559896

(US11377423, Cmpd 2A)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM559896

(US11377423, Cmpd 2A)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50457079

(CS-055 | CS055 | Chidamide | Epidaza in China | HB...)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cccnc2)cc1 Show InChI InChI=1S/C22H19FN4O2/c23-18-8-9-20(19(24)12-18)27-22(29)17-6-3-16(4-7-17)14-26-21(28)10-5-15-2-1-11-25-13-15/h1-13H,14,24H2,(H,26,28)(H,27,29)/b10-5+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM559897

(US11377423, Cmpd 3A)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cccnc2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM559897

(US11377423, Cmpd 3A)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cccnc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM24624

(CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM24624

(CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CNc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C23H20N6O/c24-19-5-1-2-6-21(19)28-22(30)17-9-7-16(8-10-17)14-27-23-26-13-11-20(29-23)18-4-3-12-25-15-18/h1-13,15H,14,24H2,(H,28,30)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM559894

(US11377423, Cmpd 1A)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNC(=O)OCc2cccnc2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50457079

(CS-055 | CS055 | Chidamide | Epidaza in China | HB...)Show SMILES Nc1cc(F)ccc1NC(=O)c1ccc(CNC(=O)\C=C\c2cccnc2)cc1 Show InChI InChI=1S/C22H19FN4O2/c23-18-8-9-20(19(24)12-18)27-22(29)17-6-3-16(4-7-17)14-26-21(28)10-5-15-2-1-11-25-13-15/h1-13H,14,24H2,(H,26,28)(H,27,29)/b10-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM178100

(BRD3308 | US11377423, Cmpd 1)Show InChI InChI=1S/C15H14FN3O2/c1-9(20)18-12-5-2-10(3-6-12)15(21)19-14-7-4-11(16)8-13(14)17/h2-8H,17H2,1H3,(H,18,20)(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM178100

(BRD3308 | US11377423, Cmpd 1)Show InChI InChI=1S/C15H14FN3O2/c1-9(20)18-12-5-2-10(3-6-12)15(21)19-14-7-4-11(16)8-13(14)17/h2-8H,17H2,1H3,(H,18,20)(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM559883
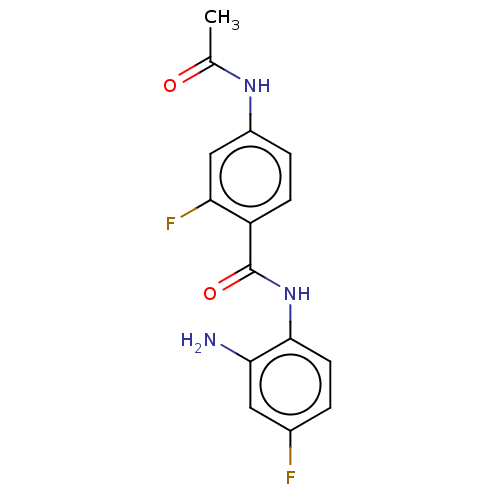
(US11377423, Compound 6) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM559887
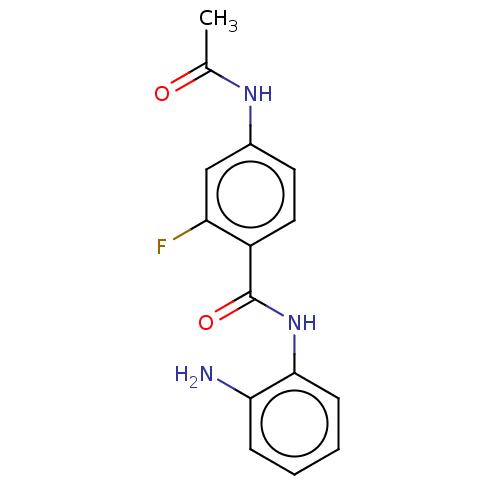
(US11377423, Compound 12) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM559887

(US11377423, Compound 12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The following non-trypsin coupled in-vitro HDAC enzymatic endpoint assay was used to assay the compounds of the invention. Below is a standardized pr... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FX7DPJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data