

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4707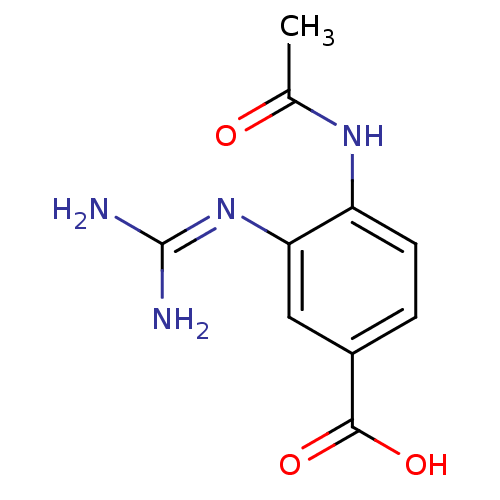 (3-(2,2-diaminoimino)-4-methylcarboxamidobenzoate |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against B/Lee/40 Influenza B Neuraminidase. | J Med Chem 42: 2332-43 (1999) Article DOI: 10.1021/jm980707k BindingDB Entry DOI: 10.7270/Q25B01NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM5266 ((2S,3S,4R)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5265 ((2S,3S,4R)-4-carbamimidamido-3-acetamido-2-[(1R,2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM5265 ((2S,3S,4R)-4-carbamimidamido-3-acetamido-2-[(1R,2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017371 (1-[4-(3-{4-[Bis-(4-fluoro-phenyl)-hydroxy-methyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225193 (CHEMBL432463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM76863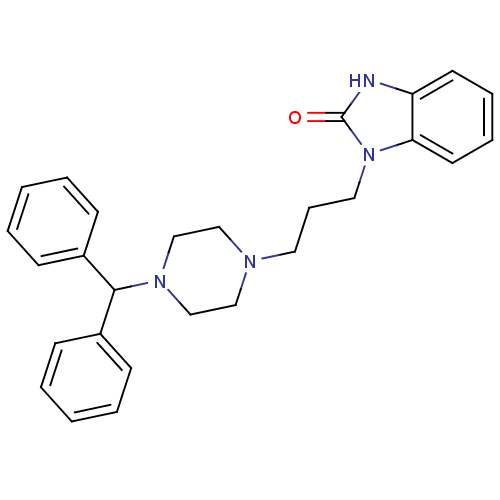 (3-[3-(4-benzhydrylpiperazin-1-yl)propyl]-1H-benzim...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to Histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225190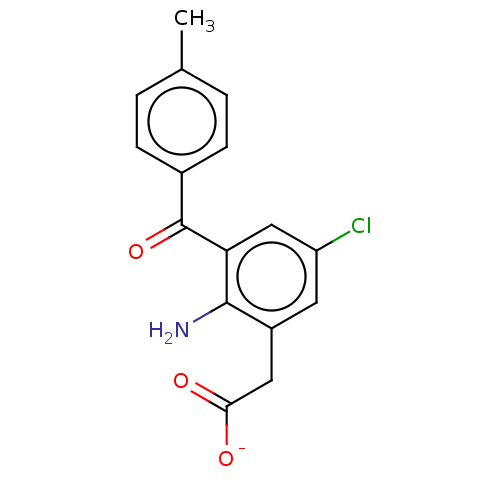 (CHEMBL28508) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225114 (CHEMBL28507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225184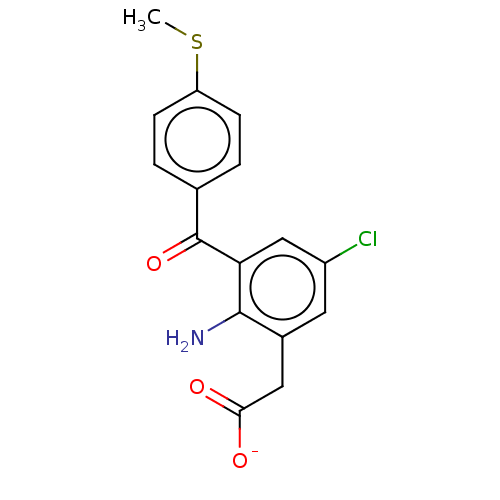 (CHEMBL28976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225186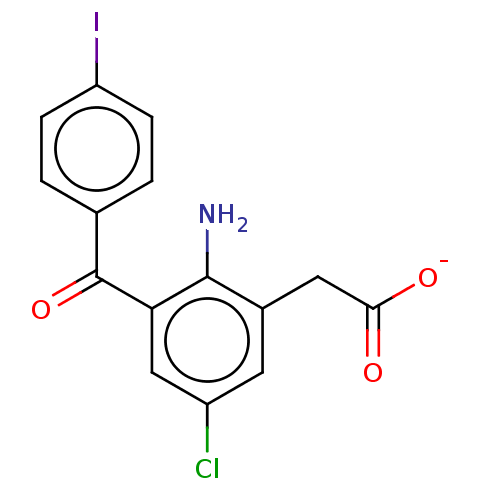 (CHEMBL281012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225122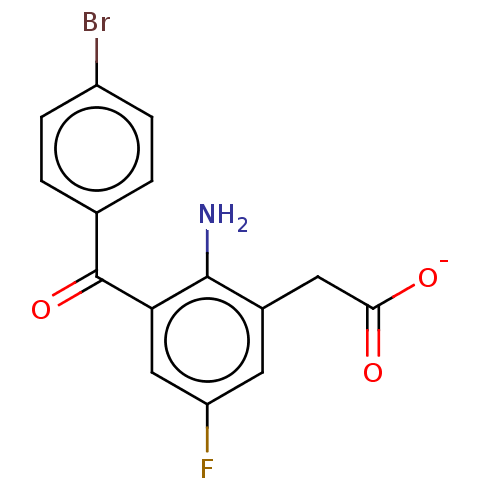 (CHEMBL29047) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017374 (1-(4-{3-[4-(Hydroxy-diphenyl-methyl)-piperidin-1-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50078329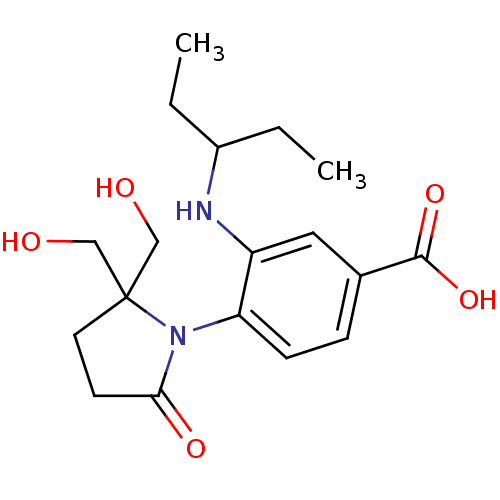 (1-[4-CARBOXY-2-(3-PENTYLAMINO)PHENYL]-5,5'-DI(HYDR...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against H1N9 Influenza A Neuraminidase. | J Med Chem 42: 2332-43 (1999) Article DOI: 10.1021/jm980707k BindingDB Entry DOI: 10.7270/Q25B01NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225136 (CHEMBL538408) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225141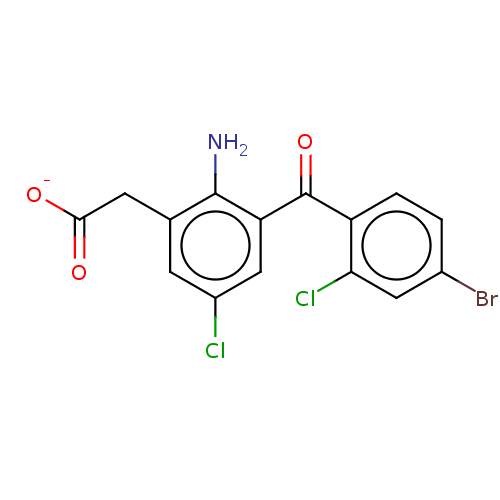 (CHEMBL418035) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017372 (4-(3-{4-[Bis-(4-fluoro-phenyl)-hydroxy-methyl]-pip...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225112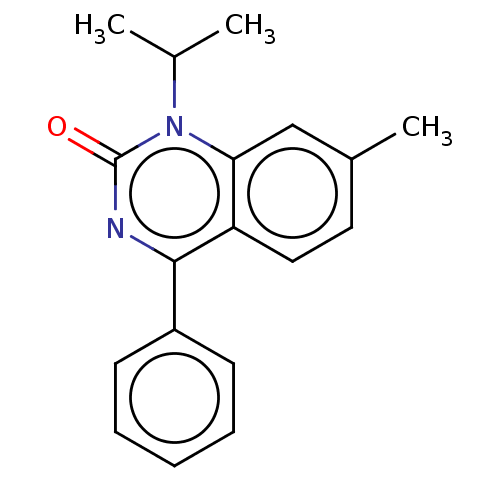 (43-715 | Arthrex | Proquazone) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description -Log (Ki) value for butyrylcholinesterase by inhibiting DFP | J Med Chem 27: 1317-21 (1984) BindingDB Entry DOI: 10.7270/Q25T3NPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225123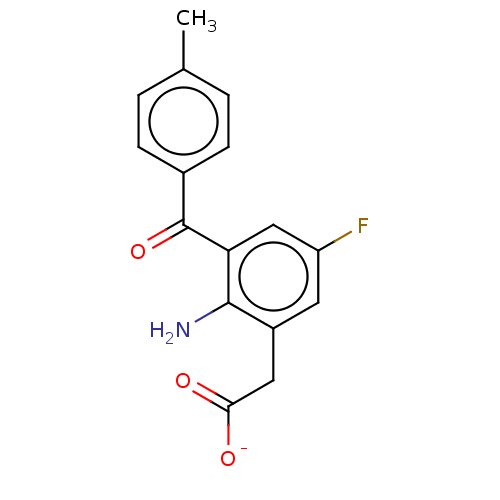 (CHEMBL28543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225195 (CHEMBL31313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225143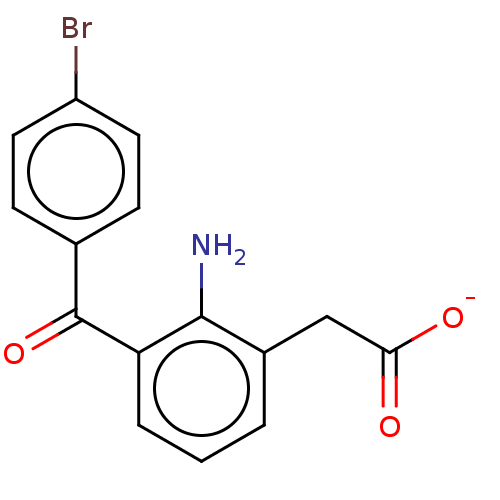 (AHR-10282B | Bromday | Bromfenac Sodium | Bromsite...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225126 (CHEMBL283267) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50224285 (AHR-5850D | Amfenac Sodium | CHEBI:75918) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase | J Med Chem 33: 2296-304 (1990) BindingDB Entry DOI: 10.7270/Q2MS3RR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50225187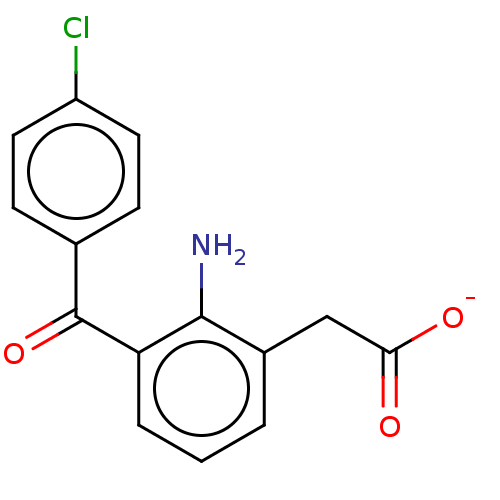 (CHEMBL28700) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase | J Med Chem 33: 2296-304 (1990) BindingDB Entry DOI: 10.7270/Q2MS3RR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225118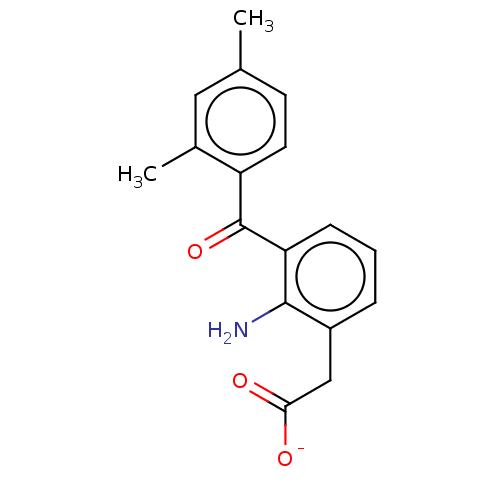 (CHEMBL436147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225121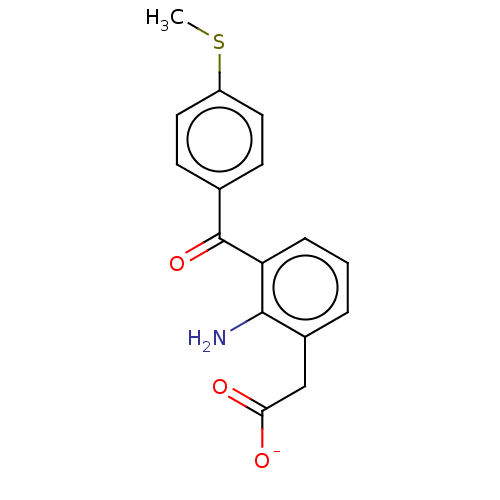 (CHEMBL286716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225134 (CHEMBL32037) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225176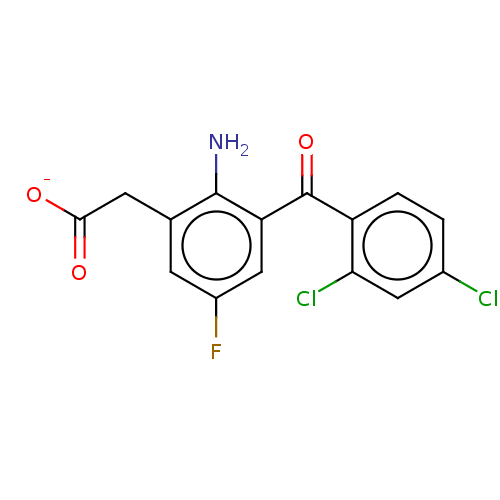 (CHEMBL29291) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225181 (CHEMBL282828) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225140 (CHEMBL28955) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225110 (Amfenac | CHEBI:75915) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description -Log (Ki) value for butyrylcholinesterase by inhibiting DFP | J Med Chem 27: 1317-21 (1984) BindingDB Entry DOI: 10.7270/Q25T3NPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50224285 (AHR-5850D | Amfenac Sodium | CHEBI:75918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of H-Ras-mediated farnesylation expressed in mouse NIH3T3 cells | J Med Chem 25: 446-51 (1982) BindingDB Entry DOI: 10.7270/Q28P62QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50224285 (AHR-5850D | Amfenac Sodium | CHEBI:75918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase | J Med Chem 33: 2296-304 (1990) BindingDB Entry DOI: 10.7270/Q2MS3RR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017376 ((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225187 (CHEMBL28700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225135 (CHEMBL284647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225130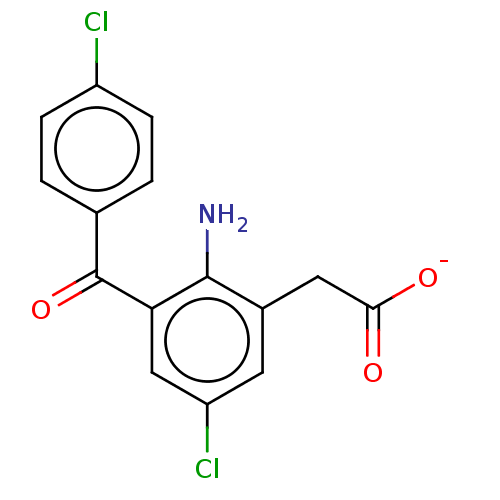 (CHEMBL27620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225117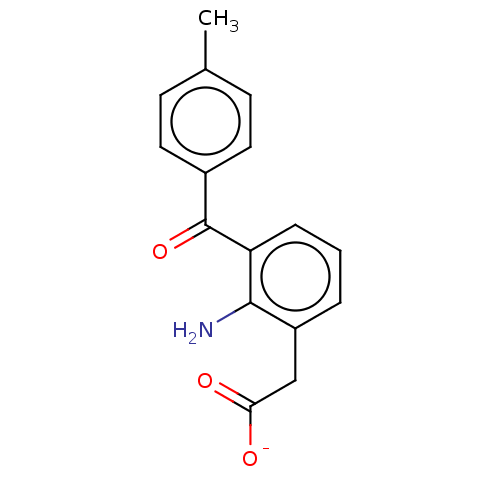 (CHEMBL28703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225185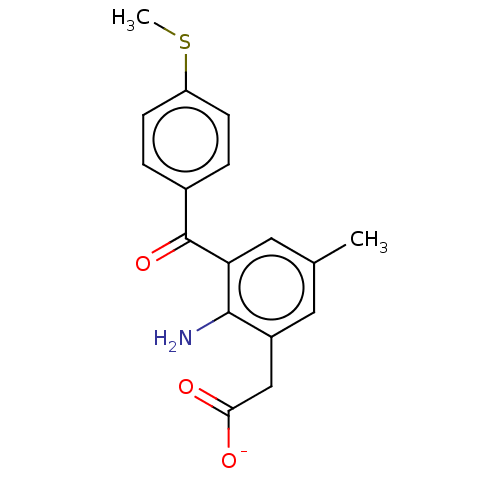 (CHEMBL28511) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50228722 (CHEMBL75896) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase | J Med Chem 33: 2296-304 (1990) BindingDB Entry DOI: 10.7270/Q2MS3RR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5266 ((2S,3S,4R)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225120 (CHEMBL28469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225178 (CHEMBL282824) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225191 (CHEMBL284879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225113 (CHEMBL441960) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225139 (CHEMBL29112) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225137 (CHEMBL283661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50225133 (CHEMBL28309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of prostaglandin G/H synthase obtained from bovine seminal vesicles. | J Med Chem 27: 1379-88 (1984) BindingDB Entry DOI: 10.7270/Q2222X0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50017373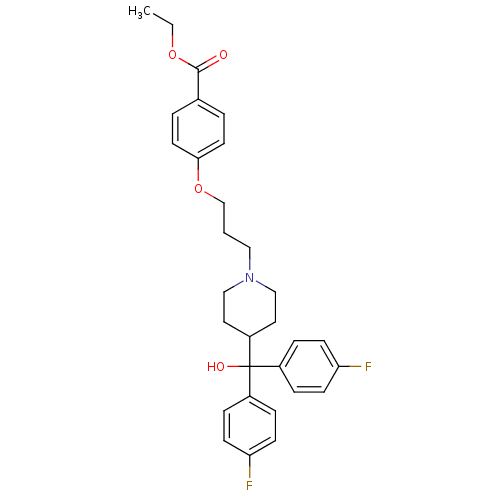 (4-(3-{4-[Bis-(4-fluoro-phenyl)-hydroxy-methyl]-pip...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 956 | n/a | n/a | n/a | n/a | n/a | n/a |
A.H. Robins Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Tritiated [3H]- mepyramine binding to histamine H1 receptor in guinea pig cerebral cortex | J Med Chem 32: 105-18 (1989) BindingDB Entry DOI: 10.7270/Q2WM1CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 168 total ) | Next | Last >> |