 Found 255 hits with Last Name = 'walters' and Initial = 'e'
Found 255 hits with Last Name = 'walters' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50483336

(CHEMBL1651153 | GRL-0476)Show SMILES [H][C@@]12CCO[C@]1([H])OCC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H40N2O8S/c1-20(2)18-31(40(34,35)23-11-9-22(36-3)10-12-23)19-26(32)25(17-21-7-5-4-6-8-21)30-29(33)39-27-14-16-38-28-24(27)13-15-37-28/h4-12,20,24-28,32H,13-19H2,1-3H3,(H,30,33)/t24-,25-,26+,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 54: 622-34 (2011)
Article DOI: 10.1021/jm1012787
BindingDB Entry DOI: 10.7270/Q2BV7KG9 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13925
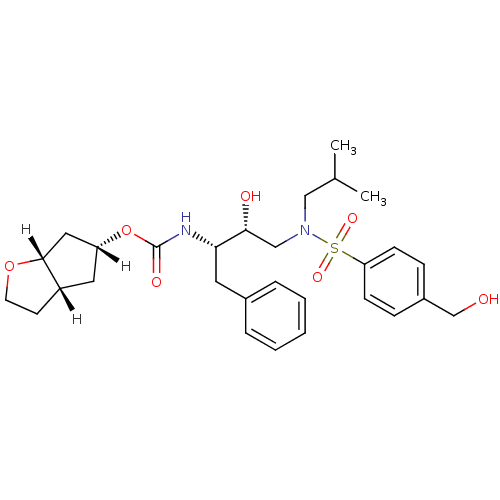
((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(CO)cc1 |r| Show InChI InChI=1S/C29H40N2O7S/c1-20(2)17-31(39(35,36)25-10-8-22(19-32)9-11-25)18-27(33)26(14-21-6-4-3-5-7-21)30-29(34)38-24-15-23-12-13-37-28(23)16-24/h3-11,20,23-24,26-28,32-33H,12-19H2,1-2H3,(H,30,34)/t23-,24+,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50483338

(CHEMBL1651155)Show SMILES [H][C@@]12CCC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(OC)cc3)[C@]1([H])CCO2 |r| Show InChI InChI=1S/C30H42N2O7S/c1-21(2)19-32(40(35,36)24-14-12-23(37-3)13-15-24)20-27(33)26(18-22-8-5-4-6-9-22)31-30(34)39-29-11-7-10-28-25(29)16-17-38-28/h4-6,8-9,12-15,21,25-29,33H,7,10-11,16-20H2,1-3H3,(H,31,34)/t25-,26+,27-,28-,29+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 54: 622-34 (2011)
Article DOI: 10.1021/jm1012787
BindingDB Entry DOI: 10.7270/Q2BV7KG9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481584
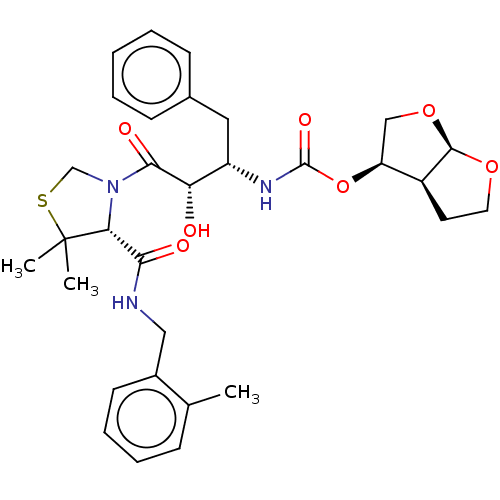
(CHEMBL589988 | GRL-0355)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C31H39N3O7S/c1-19-9-7-8-12-21(19)16-32-27(36)26-31(2,3)42-18-34(26)28(37)25(35)23(15-20-10-5-4-6-11-20)33-30(38)41-24-17-40-29-22(24)13-14-39-29/h4-12,22-26,29,35H,13-18H2,1-3H3,(H,32,36)(H,33,38)/t22-,23-,24-,25-,26+,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50483334

(CHEMBL1651160)Show SMILES [H][C@@]12CCO[C@]1([H])OCC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C28H39N3O7S/c1-19(2)17-31(39(34,35)22-10-8-21(29)9-11-22)18-25(32)24(16-20-6-4-3-5-7-20)30-28(33)38-26-13-15-37-27-23(26)12-14-36-27/h3-11,19,23-27,32H,12-18,29H2,1-2H3,(H,30,33)/t23-,24-,25+,26-,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 54: 622-34 (2011)
Article DOI: 10.1021/jm1012787
BindingDB Entry DOI: 10.7270/Q2BV7KG9 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9236

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C28H38N2O8S/c1-19(2)16-30(39(33,34)22-11-9-21(35-3)10-12-22)17-25(31)24(15-20-7-5-4-6-8-20)29-28(32)38-26-18-37-27-23(26)13-14-36-27/h4-12,19,23-27,31H,13-18H2,1-3H3,(H,29,32)/t23-,24-,25+,26-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 12: 1993-6 (2002)
Article DOI: 10.1016/s0960-894x(02)00300-1
BindingDB Entry DOI: 10.7270/Q2SJ1HS5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay |
Bioorg Med Chem Lett 29: 2565-2570 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.006
BindingDB Entry DOI: 10.7270/Q2JM2F1C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50483337

(CHEMBL1651154)Show SMILES [H][C@]12CCO[C@@]1([H])OCC[C@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H40N2O8S/c1-20(2)18-31(40(34,35)23-11-9-22(36-3)10-12-23)19-26(32)25(17-21-7-5-4-6-8-21)30-29(33)39-27-14-16-38-28-24(27)13-15-37-28/h4-12,20,24-28,32H,13-19H2,1-3H3,(H,30,33)/t24-,25+,26-,27-,28+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 54: 622-34 (2011)
Article DOI: 10.1021/jm1012787
BindingDB Entry DOI: 10.7270/Q2BV7KG9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50483335

(CHEMBL1651161)Show SMILES [H][C@@]12CCO[C@]1([H])OCC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(CO)cc1 |r| Show InChI InChI=1S/C29H40N2O8S/c1-20(2)17-31(40(35,36)23-10-8-22(19-32)9-11-23)18-26(33)25(16-21-6-4-3-5-7-21)30-29(34)39-27-13-15-38-28-24(27)12-14-37-28/h3-11,20,24-28,32-33H,12-19H2,1-2H3,(H,30,34)/t24-,25-,26+,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 54: 622-34 (2011)
Article DOI: 10.1021/jm1012787
BindingDB Entry DOI: 10.7270/Q2BV7KG9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50483341

(CHEMBL1651159)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H](C2)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H40N2O8S/c1-20(2)17-31(40(34,35)25-11-9-23(36-3)10-12-25)18-27(32)26(15-21-7-5-4-6-8-21)30-29(33)39-24-16-22-13-14-37-28(22)38-19-24/h4-12,20,22,24,26-28,32H,13-19H2,1-3H3,(H,30,33)/t22-,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 54: 622-34 (2011)
Article DOI: 10.1021/jm1012787
BindingDB Entry DOI: 10.7270/Q2BV7KG9 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13924
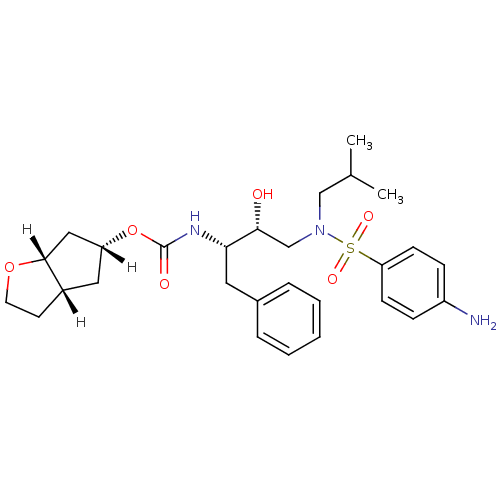
((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C28H39N3O6S/c1-19(2)17-31(38(34,35)24-10-8-22(29)9-11-24)18-26(32)25(14-20-6-4-3-5-7-20)30-28(33)37-23-15-21-12-13-36-27(21)16-23/h3-11,19,21,23,25-27,32H,12-18,29H2,1-2H3,(H,30,33)/t21-,23+,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481586
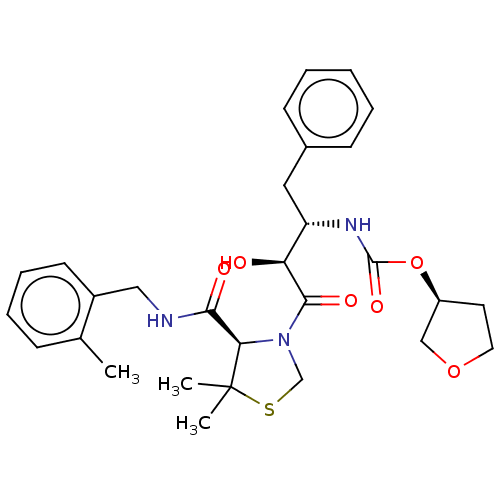
(CHEMBL604931)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1 |r| Show InChI InChI=1S/C29H37N3O6S/c1-19-9-7-8-12-21(19)16-30-26(34)25-29(2,3)39-18-32(25)27(35)24(33)23(15-20-10-5-4-6-11-20)31-28(36)38-22-13-14-37-17-22/h4-12,22-25,33H,13-18H2,1-3H3,(H,30,34)(H,31,36)/t22-,23-,24-,25+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481579

(CHEMBL601049)Show SMILES [H][C@@]12CCO[C@]1([H])C[C@@H](C2)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C32H41N3O6S/c1-20-9-7-8-12-23(20)18-33-29(37)28-32(2,3)42-19-35(28)30(38)27(36)25(15-21-10-5-4-6-11-21)34-31(39)41-24-16-22-13-14-40-26(22)17-24/h4-12,22,24-28,36H,13-19H2,1-3H3,(H,33,37)(H,34,39)/t22-,24+,25-,26+,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481582

(CHEMBL601052)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccc(CO)cc1C |r| Show InChI InChI=1S/C32H41N3O8S/c1-19-13-21(16-36)9-10-22(19)15-33-28(38)27-32(2,3)44-18-35(27)29(39)26(37)24(14-20-7-5-4-6-8-20)34-31(40)43-25-17-42-30-23(25)11-12-41-30/h4-10,13,23-27,30,36-37H,11-12,14-18H2,1-3H3,(H,33,38)(H,34,40)/t23-,24-,25-,26-,27+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50522184

(CHEMBL4444017)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)Nc1c(N(C)[C@@H]2CCOC2)c(=O)c1=O |r| Show InChI InChI=1S/C30H39N3O7S/c1-20(2)17-33(41(37,38)24-12-10-23(39-4)11-13-24)18-26(34)25(16-21-8-6-5-7-9-21)31-27-28(30(36)29(27)35)32(3)22-14-15-40-19-22/h5-13,20,22,25-26,31,34H,14-19H2,1-4H3/t22-,25+,26-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay |
Bioorg Med Chem Lett 29: 2565-2570 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.006
BindingDB Entry DOI: 10.7270/Q2JM2F1C |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481583

(CHEMBL601050)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])OCO2)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C31H39N3O7S/c1-19-9-7-8-12-21(19)16-32-28(36)27-31(2,3)42-17-34(27)29(37)26(35)23(13-20-10-5-4-6-11-20)33-30(38)41-22-14-24-25(15-22)40-18-39-24/h4-12,22-27,35H,13-18H2,1-3H3,(H,32,36)(H,33,38)/t22-,23-,24+,25-,26-,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9264

((2R)-2-hydroxy-2-[(10S)-16-hydroxy-12-oxo-3,4,5,6,...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@@H]1CCCCCCCCOc2c(O)cccc2C(=O)N1 |r| Show InChI InChI=1S/C29H42N2O7S/c1-21(2)19-31(39(35,36)23-16-14-22(37-3)15-17-23)20-27(33)25-12-8-6-4-5-7-9-18-38-28-24(29(34)30-25)11-10-13-26(28)32/h10-11,13-17,21,25,27,32-33H,4-9,12,18-20H2,1-3H3,(H,30,34)/t25-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
J Med Chem 48: 3576-85 (2005)
Article DOI: 10.1021/jm050019i
BindingDB Entry DOI: 10.7270/Q2NS0S39 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481587

(CHEMBL599785)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@@H]1CCOCOC1 |r| Show InChI InChI=1S/C30H39N3O7S/c1-20-9-7-8-12-22(20)16-31-27(35)26-30(2,3)41-18-33(26)28(36)25(34)24(15-21-10-5-4-6-11-21)32-29(37)40-23-13-14-38-19-39-17-23/h4-12,23-26,34H,13-19H2,1-3H3,(H,31,35)(H,32,37)/t23-,24+,25+,26-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481581
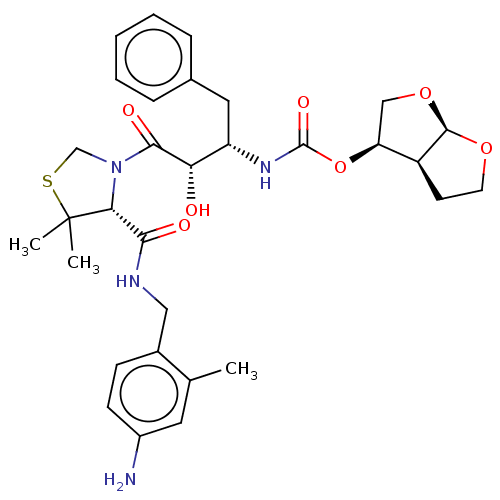
(CHEMBL601051)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccc(N)cc1C |r| Show InChI InChI=1S/C31H40N4O7S/c1-18-13-21(32)10-9-20(18)15-33-27(37)26-31(2,3)43-17-35(26)28(38)25(36)23(14-19-7-5-4-6-8-19)34-30(39)42-24-16-41-29-22(24)11-12-40-29/h4-10,13,22-26,29,36H,11-12,14-17,32H2,1-3H3,(H,33,37)(H,34,39)/t22-,23-,24-,25-,26+,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317217
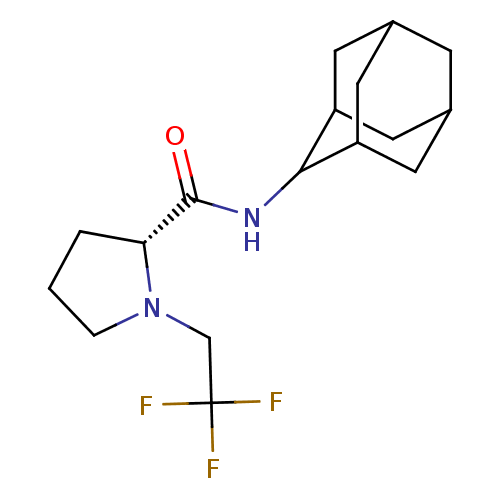
((2R)-N-(adamantan-2-yl)-1-(2,2,2-trifluoroethyl)py...)Show SMILES FC(F)(F)CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:9.10,TLB:19:18:22:15.14.13,19:14:17.18.20:22,THB:12:13:17.18.20:22,13:14:17:20.21.22,13:21:17:15.19.14,(27.97,-20.63,;27.2,-21.96,;25.66,-21.96,;26.42,-20.62,;27.97,-23.29,;27.07,-24.54,;25.53,-24.56,;25.07,-26.02,;26.31,-26.92,;27.52,-25.95,;29.03,-26.46,;30.37,-25.71,;29,-28,;27.66,-28.74,;27.65,-30.27,;26.25,-30.63,;24.91,-30.13,;23.71,-31.41,;25.22,-30.99,;26.63,-31.56,;25.21,-29.4,;26.26,-28.16,;24.9,-28.65,)| Show InChI InChI=1S/C17H25F3N2O/c18-17(19,20)9-22-3-1-2-14(22)16(23)21-15-12-5-10-4-11(7-12)8-13(15)6-10/h10-15H,1-9H2,(H,21,23)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317221
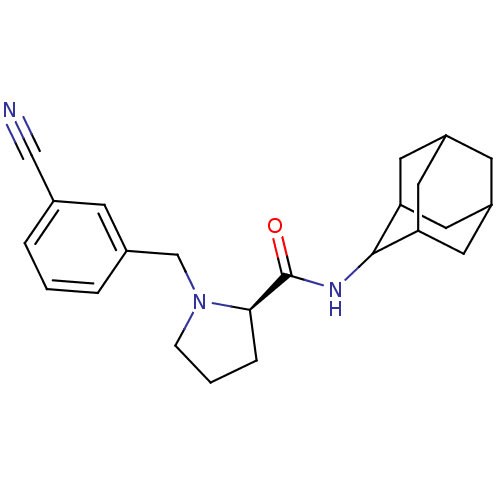
((2R)-N-(adamantan-2-yl)-1-[(3-cyanophenyl)methyl]p...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1Cc1cccc(c1)C#N |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(29.81,-42.16,;28.46,-42.91,;28.44,-44.45,;27.09,-45.2,;27.08,-46.73,;25.68,-47.08,;24.34,-46.59,;23.15,-47.87,;24.65,-47.44,;26.06,-48.01,;24.64,-45.86,;25.69,-44.62,;24.33,-45.1,;26.95,-42.4,;25.74,-43.38,;24.5,-42.47,;24.97,-41.02,;26.5,-41,;27.4,-39.75,;26.63,-38.41,;25.1,-38.41,;24.33,-37.08,;25.11,-35.74,;26.65,-35.75,;27.41,-37.09,;27.43,-34.42,;28.2,-33.09,)| Show InChI InChI=1S/C23H29N3O/c24-13-15-3-1-4-16(7-15)14-26-6-2-5-21(26)23(27)25-22-19-9-17-8-18(11-19)12-20(22)10-17/h1,3-4,7,17-22H,2,5-6,8-12,14H2,(H,25,27)/t17?,18?,19?,20?,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317211
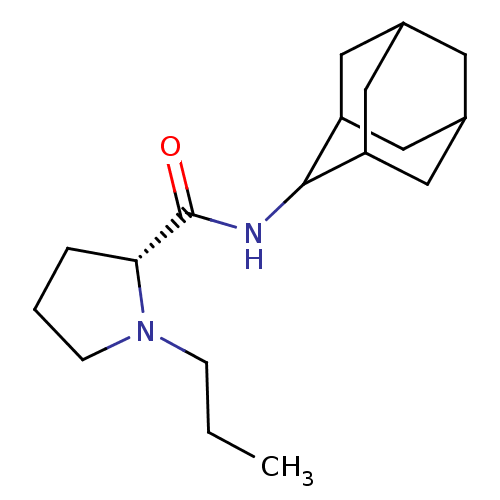
((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317224
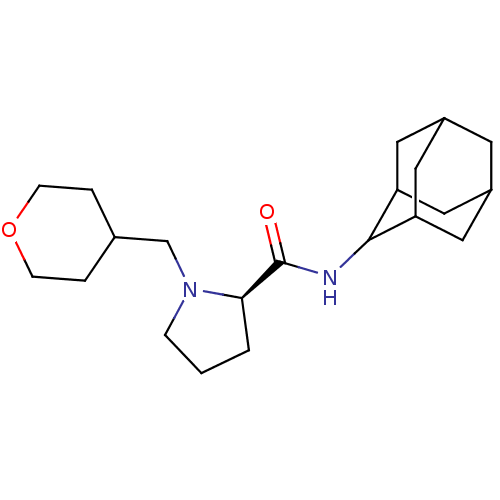
((2R)-N-(adamantan-2-yl)-1-(oxan-4-ylmethyl)pyrroli...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCOCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(16.19,2.36,;14.84,1.61,;14.82,.07,;13.47,-.68,;13.46,-2.21,;12.06,-2.57,;10.72,-2.07,;9.51,-3.36,;11.02,-2.93,;12.44,-3.5,;11.01,-1.34,;12.06,-.09,;10.7,-.58,;13.33,2.12,;12.11,1.14,;10.87,2.05,;11.33,3.52,;12.87,3.53,;13.64,4.87,;15.19,4.87,;15.96,3.52,;17.5,3.52,;18.28,4.86,;17.51,6.2,;15.96,6.21,)| Show InChI InChI=1S/C21H34N2O2/c24-21(19-2-1-5-23(19)13-14-3-6-25-7-4-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317218
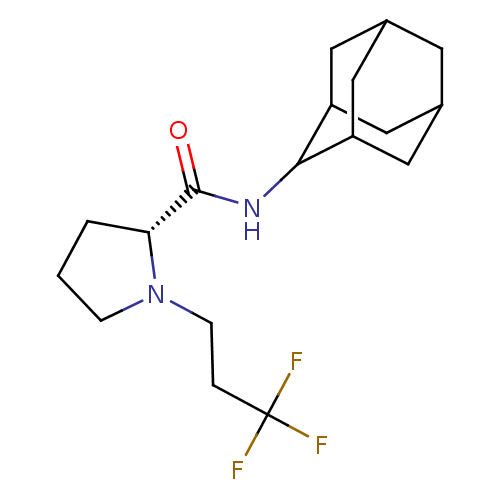
((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...)Show SMILES FC(F)(F)CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(-5.42,-32.99,;-4.79,-34.4,;-3.25,-34.55,;-4.03,-33.05,;-5.69,-35.65,;-5.05,-37.05,;-5.95,-38.3,;-7.48,-38.31,;-7.95,-39.77,;-6.71,-40.68,;-5.5,-39.7,;-3.99,-40.21,;-2.65,-39.46,;-4.02,-41.75,;-5.36,-42.5,;-5.37,-44.03,;-6.77,-44.38,;-8.11,-43.89,;-9.31,-45.17,;-7.8,-44.74,;-6.39,-45.31,;-7.81,-43.16,;-6.76,-41.92,;-8.12,-42.4,)| Show InChI InChI=1S/C18H27F3N2O/c19-18(20,21)3-5-23-4-1-2-15(23)17(24)22-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,1-10H2,(H,22,24)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9262
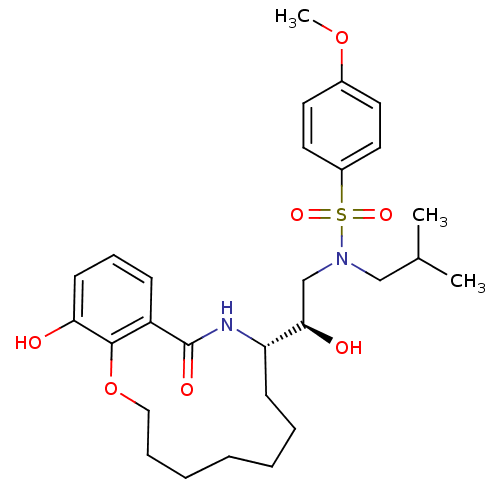
((2R)-2-hydroxy-2-[(9S)-15-hydroxy-11-oxo-2,3,4,5,6...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@@H]1CCCCCCCOc2c(O)cccc2C(=O)N1 |r| Show InChI InChI=1S/C28H40N2O7S/c1-20(2)18-30(38(34,35)22-15-13-21(36-3)14-16-22)19-26(32)24-11-7-5-4-6-8-17-37-27-23(28(33)29-24)10-9-12-25(27)31/h9-10,12-16,20,24,26,31-32H,4-8,11,17-19H2,1-3H3,(H,29,33)/t24-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
J Med Chem 48: 3576-85 (2005)
Article DOI: 10.1021/jm050019i
BindingDB Entry DOI: 10.7270/Q2NS0S39 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317213
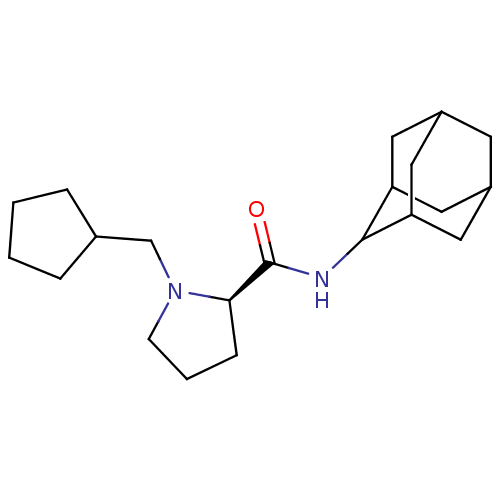
((2R)-N-(adamantan-2-yl)-1-(cyclopentylmethyl)pyrro...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(25.66,-12.64,;24.31,-13.39,;24.29,-14.93,;22.94,-15.67,;22.93,-17.2,;21.53,-17.56,;20.2,-17.06,;19,-18.34,;20.5,-17.92,;21.92,-18.49,;20.5,-16.33,;21.54,-15.09,;20.18,-15.58,;22.8,-12.88,;21.59,-13.85,;20.36,-12.95,;20.82,-11.49,;22.35,-11.47,;23.25,-10.22,;22.62,-8.82,;23.38,-7.49,;22.34,-6.35,;20.94,-6.98,;21.1,-8.51,)| Show InChI InChI=1S/C21H34N2O/c24-21(19-6-3-7-23(19)13-14-4-1-2-5-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317223
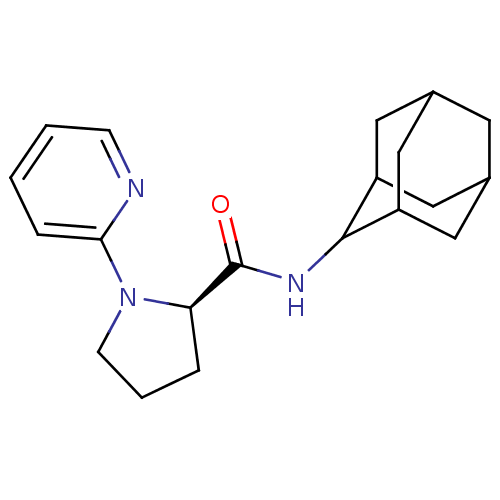
((2R)-N-(adamantan-2-yl)-1-(pyridin-2-yl)pyrrolidin...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1c1ccccn1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(6.04,2.3,;4.69,1.55,;4.66,.01,;3.32,-.73,;3.31,-2.26,;1.91,-2.62,;.57,-2.12,;-.63,-3.4,;.88,-2.98,;2.29,-3.54,;.87,-1.39,;1.92,-.15,;.56,-.63,;3.18,2.06,;1.97,1.09,;.73,1.99,;1.2,3.45,;2.72,3.47,;4.03,4.27,;4,5.82,;5.31,6.62,;6.67,5.87,;6.7,4.35,;5.39,3.54,)| Show InChI InChI=1S/C20H27N3O/c24-20(17-4-3-7-23(17)18-5-1-2-6-21-18)22-19-15-9-13-8-14(11-15)12-16(19)10-13/h1-2,5-6,13-17,19H,3-4,7-12H2,(H,22,24)/t13?,14?,15?,16?,17-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
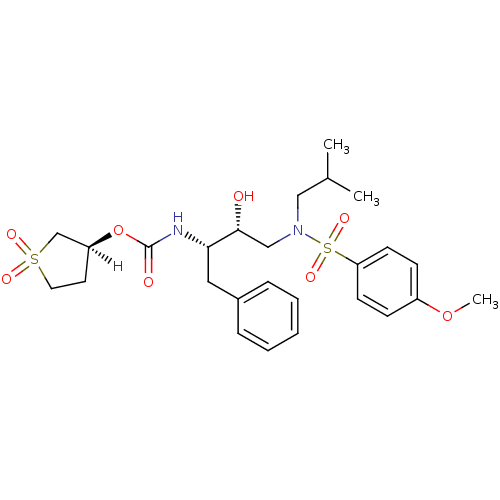
(Human immunodeficiency virus type 1) | BDBM9270
((3S)-1,1-dioxo--thiolan-3-yl N-[(2S,3R)-3-hydroxy-...)Show SMILES [H][C@@]1(CCS(=O)(=O)C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C26H36N2O8S2/c1-19(2)16-28(38(33,34)23-11-9-21(35-3)10-12-23)17-25(29)24(15-20-7-5-4-6-8-20)27-26(30)36-22-13-14-37(31,32)18-22/h4-12,19,22,24-25,29H,13-18H2,1-3H3,(H,27,30)/t22-,24-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 8: 687-90 (1998)
Article DOI: 10.1016/s0960-894x(98)00098-5
BindingDB Entry DOI: 10.7270/Q2J101DQ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
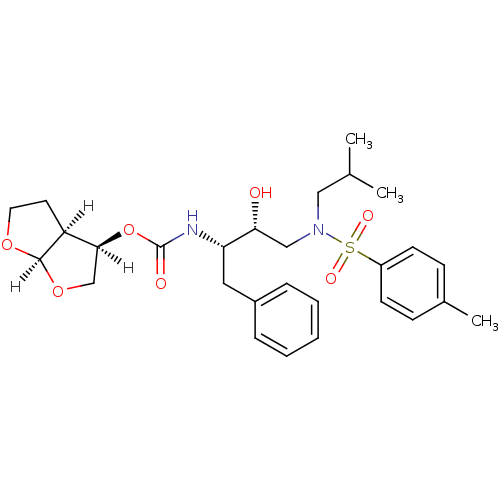
(Human immunodeficiency virus type 1) | BDBM9277
((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C28H38N2O7S/c1-19(2)16-30(38(33,34)22-11-9-20(3)10-12-22)17-25(31)24(15-21-7-5-4-6-8-21)29-28(32)37-26-18-36-27-23(26)13-14-35-27/h4-12,19,23-27,31H,13-18H2,1-3H3,(H,29,32)/t23-,24-,25+,26-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 8: 687-90 (1998)
Article DOI: 10.1016/s0960-894x(98)00098-5
BindingDB Entry DOI: 10.7270/Q2J101DQ |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50483342
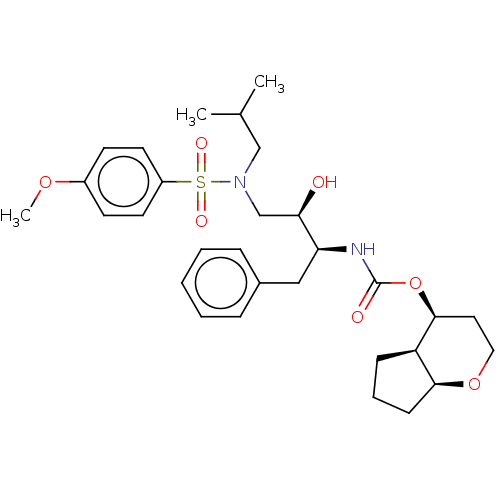
(CHEMBL1651156)Show SMILES [H][C@]12CCC[C@@]1([H])[C@H](CCO2)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H42N2O7S/c1-21(2)19-32(40(35,36)24-14-12-23(37-3)13-15-24)20-27(33)26(18-22-8-5-4-6-9-22)31-30(34)39-29-16-17-38-28-11-7-10-25(28)29/h4-6,8-9,12-15,21,25-29,33H,7,10-11,16-20H2,1-3H3,(H,31,34)/t25-,26+,27-,28+,29+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 54: 622-34 (2011)
Article DOI: 10.1021/jm1012787
BindingDB Entry DOI: 10.7270/Q2BV7KG9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317211

((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209
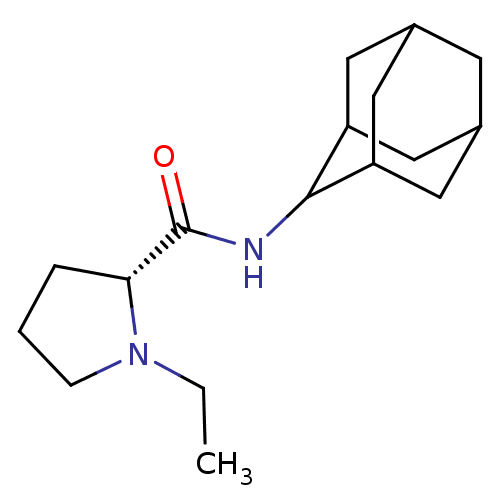
((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against HIV protease |
Bioorg Med Chem Lett 8: 979-82 (1999)
BindingDB Entry DOI: 10.7270/Q2D79BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9271
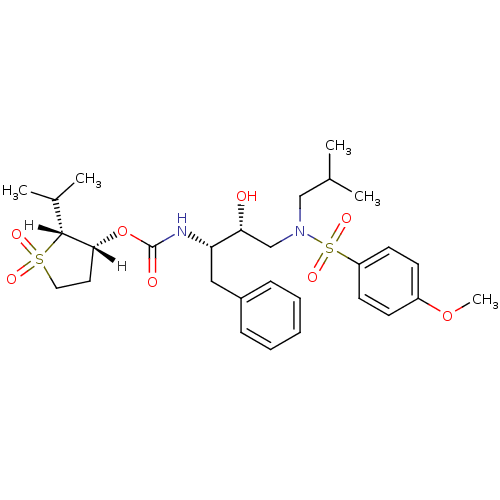
((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...)Show SMILES [H][C@]1(CCS(=O)(=O)[C@]1([H])C(C)C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H42N2O8S2/c1-20(2)18-31(41(36,37)24-13-11-23(38-5)12-14-24)19-26(32)25(17-22-9-7-6-8-10-22)30-29(33)39-27-15-16-40(34,35)28(27)21(3)4/h6-14,20-21,25-28,32H,15-19H2,1-5H3,(H,30,33)/t25-,26+,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 8: 687-90 (1998)
Article DOI: 10.1016/s0960-894x(98)00098-5
BindingDB Entry DOI: 10.7270/Q2J101DQ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9272
((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...)Show SMILES [H][C@]1(CCS(=O)(=O)[C@]1([H])C(C)C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C28H41N3O7S2/c1-19(2)17-31(40(36,37)23-12-10-22(29)11-13-23)18-25(32)24(16-21-8-6-5-7-9-21)30-28(33)38-26-14-15-39(34,35)27(26)20(3)4/h5-13,19-20,24-27,32H,14-18,29H2,1-4H3,(H,30,33)/t24-,25+,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 8: 687-90 (1998)
Article DOI: 10.1016/s0960-894x(98)00098-5
BindingDB Entry DOI: 10.7270/Q2J101DQ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
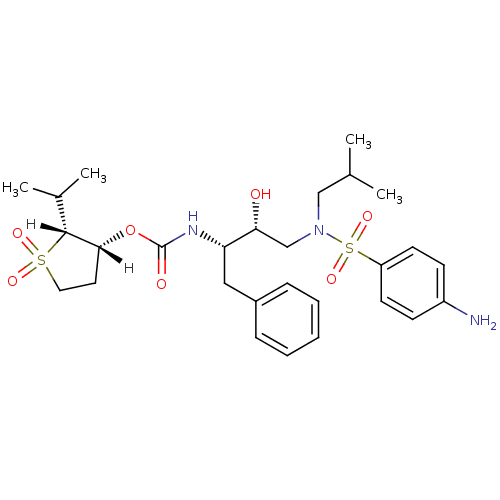
(Human immunodeficiency virus type 1) | BDBM9273
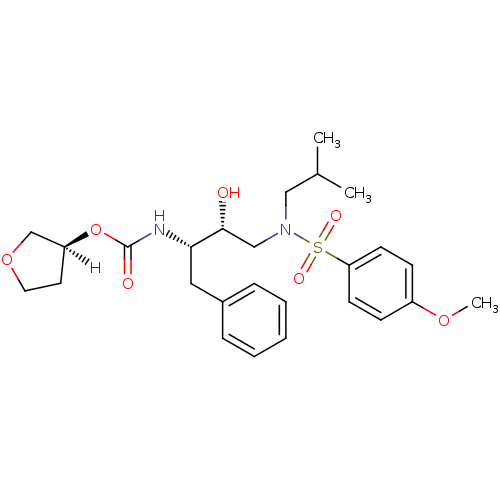
((3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[(4-methox...)Show SMILES [H][C@@]1(CCOC1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C26H36N2O7S/c1-19(2)16-28(36(31,32)23-11-9-21(33-3)10-12-23)17-25(29)24(15-20-7-5-4-6-8-20)27-26(30)35-22-13-14-34-18-22/h4-12,19,22,24-25,29H,13-18H2,1-3H3,(H,27,30)/t22-,24-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 8: 687-90 (1998)
Article DOI: 10.1016/s0960-894x(98)00098-5
BindingDB Entry DOI: 10.7270/Q2J101DQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317212
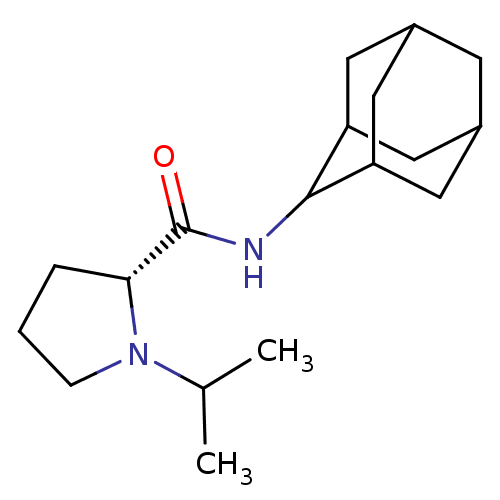
((2R)-N-(adamantan-2-yl)-1-(propan-2-yl)pyrrolidine...)Show SMILES CC(C)N1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(12.99,-6.92,;13.62,-8.33,;15.15,-8.49,;12.71,-9.58,;11.18,-9.59,;10.72,-11.05,;11.95,-11.95,;13.17,-10.98,;14.67,-11.49,;16.02,-10.74,;14.65,-13.03,;13.3,-13.78,;13.3,-15.31,;11.9,-15.66,;10.56,-15.17,;9.36,-16.45,;10.87,-16.02,;12.28,-16.59,;10.86,-14.44,;11.9,-13.19,;10.55,-13.68,)| Show InChI InChI=1S/C18H30N2O/c1-11(2)20-5-3-4-16(20)18(21)19-17-14-7-12-6-13(9-14)10-15(17)8-12/h11-17H,3-10H2,1-2H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481578

(CHEMBL589989)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccc(OC)cc1C |r| Show InChI InChI=1S/C32H41N3O8S/c1-19-14-22(40-4)11-10-21(19)16-33-28(37)27-32(2,3)44-18-35(27)29(38)26(36)24(15-20-8-6-5-7-9-20)34-31(39)43-25-17-42-30-23(25)12-13-41-30/h5-11,14,23-27,30,36H,12-13,15-18H2,1-4H3,(H,33,37)(H,34,39)/t23-,24-,25-,26-,27+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM563
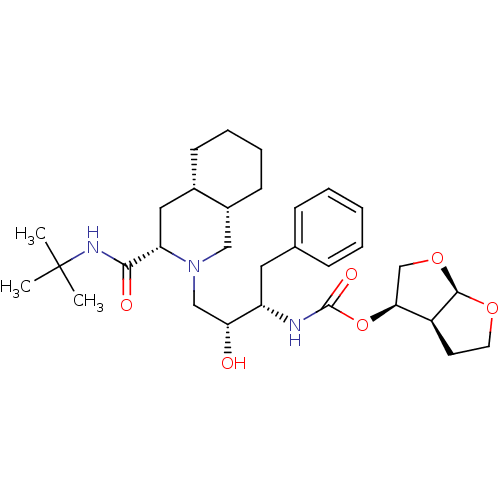
((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl (1S,2R...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CO[C@H]2OCC[C@@H]12 |r| Show InChI InChI=1S/C31H47N3O6/c1-31(2,3)33-28(36)25-16-21-11-7-8-12-22(21)17-34(25)18-26(35)24(15-20-9-5-4-6-10-20)32-30(37)40-27-19-39-29-23(27)13-14-38-29/h4-6,9-10,21-27,29,35H,7-8,11-19H2,1-3H3,(H,32,37)(H,33,36)/t21-,22+,23-,24-,25-,26+,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 12: 1993-6 (2002)
Article DOI: 10.1016/s0960-894x(02)00300-1
BindingDB Entry DOI: 10.7270/Q2SJ1HS5 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9266
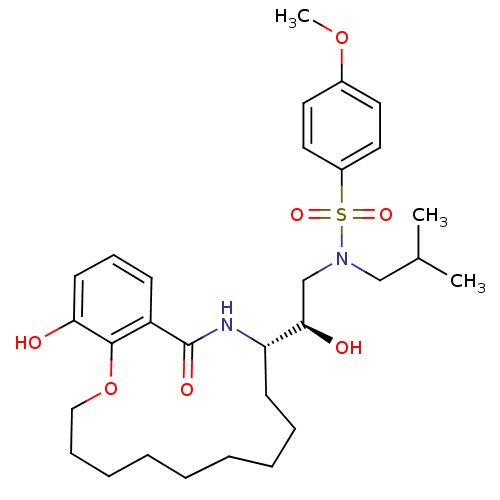
((2R)-2-hydroxy-2-[(11S)-17-hydroxy-13-oxo-2,3,4,5,...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@@H]1CCCCCCCCCOc2c(O)cccc2C(=O)N1 |r| Show InChI InChI=1S/C30H44N2O7S/c1-22(2)20-32(40(36,37)24-17-15-23(38-3)16-18-24)21-28(34)26-13-9-7-5-4-6-8-10-19-39-29-25(30(35)31-26)12-11-14-27(29)33/h11-12,14-18,22,26,28,33-34H,4-10,13,19-21H2,1-3H3,(H,31,35)/t26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
J Med Chem 48: 3576-85 (2005)
Article DOI: 10.1021/jm050019i
BindingDB Entry DOI: 10.7270/Q2NS0S39 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9263
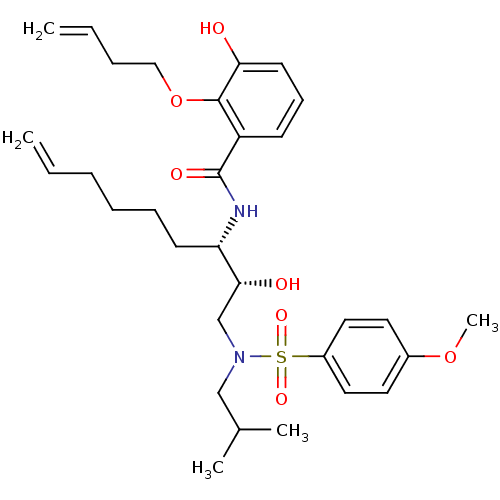
(2-(but-3-en-1-yloxy)-3-hydroxy-N-[(2R,3S)-2-hydrox...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](CCCCC=C)NC(=O)c1cccc(O)c1OCCC=C |r| Show InChI InChI=1S/C31H44N2O7S/c1-6-8-10-11-14-27(32-31(36)26-13-12-15-28(34)30(26)40-20-9-7-2)29(35)22-33(21-23(3)4)41(37,38)25-18-16-24(39-5)17-19-25/h6-7,12-13,15-19,23,27,29,34-35H,1-2,8-11,14,20-22H2,3-5H3,(H,32,36)/t27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
J Med Chem 48: 3576-85 (2005)
Article DOI: 10.1021/jm050019i
BindingDB Entry DOI: 10.7270/Q2NS0S39 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317219
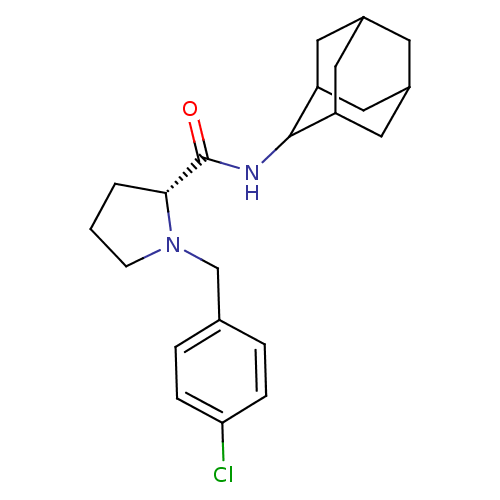
((2R)-N-(adamantan-2-yl)-1-[(4-chlorophenyl)methyl]...)Show SMILES Clc1ccc(CN2CCC[C@@H]2C(=O)NC2C3CC4CC(C3)CC2C4)cc1 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(2.87,-33.68,;3.64,-35.01,;5.18,-35.02,;5.94,-36.36,;5.16,-37.69,;5.93,-39.03,;5.02,-40.28,;3.49,-40.29,;3.03,-41.75,;4.27,-42.66,;5.48,-41.68,;6.99,-42.19,;8.34,-41.44,;6.96,-43.73,;5.62,-44.48,;5.61,-46.01,;4.21,-46.36,;2.87,-45.87,;1.67,-47.15,;3.18,-46.72,;4.59,-47.29,;3.17,-45.14,;4.22,-43.9,;2.86,-44.38,;3.63,-37.69,;2.86,-36.35,)| Show InChI InChI=1S/C22H29ClN2O/c23-19-5-3-14(4-6-19)13-25-7-1-2-20(25)22(26)24-21-17-9-15-8-16(11-17)12-18(21)10-15/h3-6,15-18,20-21H,1-2,7-13H2,(H,24,26)/t15?,16?,17?,18?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9260
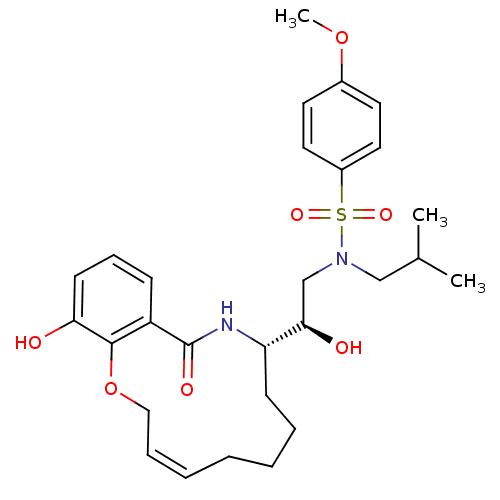
((2R)-2-hydroxy-2-[(9S)-15-hydroxy-11-oxo-2,5,6,7,8...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@@H]1CCCC\C=C/COc2c(O)cccc2C(=O)N1 |r,c:25| Show InChI InChI=1S/C28H38N2O7S/c1-20(2)18-30(38(34,35)22-15-13-21(36-3)14-16-22)19-26(32)24-11-7-5-4-6-8-17-37-27-23(28(33)29-24)10-9-12-25(27)31/h6,8-10,12-16,20,24,26,31-32H,4-5,7,11,17-19H2,1-3H3,(H,29,33)/b8-6-/t24-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
J Med Chem 48: 3576-85 (2005)
Article DOI: 10.1021/jm050019i
BindingDB Entry DOI: 10.7270/Q2NS0S39 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9278
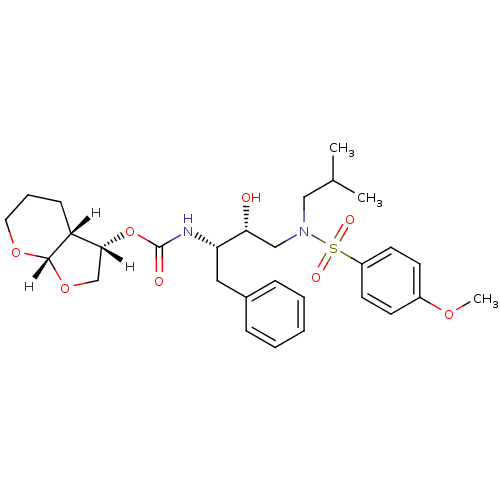
((3S,3aR,7aS)-hexahydro-2H-furo[2,3-b]pyran-3-yl N-...)Show SMILES [H][C@]1(CO[C@]2([H])OCCC[C@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H40N2O8S/c1-20(2)17-31(40(34,35)23-13-11-22(36-3)12-14-23)18-26(32)25(16-21-8-5-4-6-9-21)30-29(33)39-27-19-38-28-24(27)10-7-15-37-28/h4-6,8-9,11-14,20,24-28,32H,7,10,15-19H2,1-3H3,(H,30,33)/t24-,25+,26-,27-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 8: 687-90 (1998)
Article DOI: 10.1016/s0960-894x(98)00098-5
BindingDB Entry DOI: 10.7270/Q2J101DQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317210
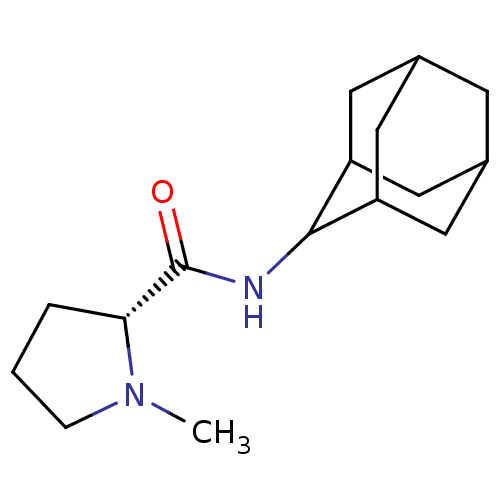
((2R)-N-(adamantan-2-yl)-1-methylpyrrolidine-2-carb...)Show SMILES CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:5.6,TLB:15:14:18:11.10.9,15:10:13.14.16:18,THB:8:9:13.14.16:18,9:10:13:16.17.18,9:17:13:11.15.10,(-4.95,-6.94,;-5.86,-8.19,;-7.4,-8.21,;-7.87,-9.67,;-6.63,-10.58,;-5.41,-9.6,;-3.89,-10.12,;-2.54,-9.36,;-3.91,-11.66,;-5.26,-12.4,;-5.27,-13.94,;-6.68,-14.3,;-8.02,-13.8,;-9.23,-15.09,;-7.71,-14.66,;-6.29,-15.23,;-7.72,-13.07,;-6.67,-11.82,;-8.03,-12.3,)| Show InChI InChI=1S/C16H26N2O/c1-18-4-2-3-14(18)16(19)17-15-12-6-10-5-11(8-12)9-13(15)7-10/h10-15H,2-9H2,1H3,(H,17,19)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317216
((2R)-N-(adamantan-2-yl)-1-(cyclohexylmethyl)pyrrol...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(18.88,-25.64,;17.53,-26.39,;17.51,-27.93,;16.16,-28.68,;16.15,-30.21,;14.75,-30.56,;13.41,-30.07,;12.22,-31.35,;13.72,-30.92,;15.13,-31.49,;13.71,-29.34,;14.76,-28.1,;13.4,-28.58,;16.02,-25.88,;14.81,-26.86,;13.57,-25.95,;14.04,-24.49,;15.57,-24.48,;16.47,-23.23,;15.7,-21.89,;16.48,-20.57,;15.71,-19.24,;14.17,-19.23,;13.4,-20.56,;14.17,-21.9,)| Show InChI InChI=1S/C22H36N2O/c25-22(20-7-4-8-24(20)14-15-5-2-1-3-6-15)23-21-18-10-16-9-17(12-18)13-19(21)11-16/h15-21H,1-14H2,(H,23,25)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9269

((3S)-thiolan-3-yl N-[(2S,3R)-3-hydroxy-4-[(4-metho...)Show SMILES [H][C@@]1(CCSC1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C26H36N2O6S2/c1-19(2)16-28(36(31,32)23-11-9-21(33-3)10-12-23)17-25(29)24(15-20-7-5-4-6-8-20)27-26(30)34-22-13-14-35-18-22/h4-12,19,22,24-25,29H,13-18H2,1-3H3,(H,27,30)/t22-,24-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | -49.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 8: 687-90 (1998)
Article DOI: 10.1016/s0960-894x(98)00098-5
BindingDB Entry DOI: 10.7270/Q2J101DQ |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481580

(CHEMBL601048)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C31H41N3O6S/c1-20-9-7-8-12-22(20)16-32-28(36)27-31(2,3)41-19-34(27)17-25(35)24(15-21-10-5-4-6-11-21)33-30(37)40-26-18-39-29-23(26)13-14-38-29/h4-12,23-27,29,35H,13-19H2,1-3H3,(H,32,36)(H,33,37)/t23-,24-,25+,26-,27+,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 20: 1241-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.123
BindingDB Entry DOI: 10.7270/Q2CJ8HB8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data