 Found 216 hits with Last Name = 'wang' and Initial = 'ch'
Found 216 hits with Last Name = 'wang' and Initial = 'ch' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300041
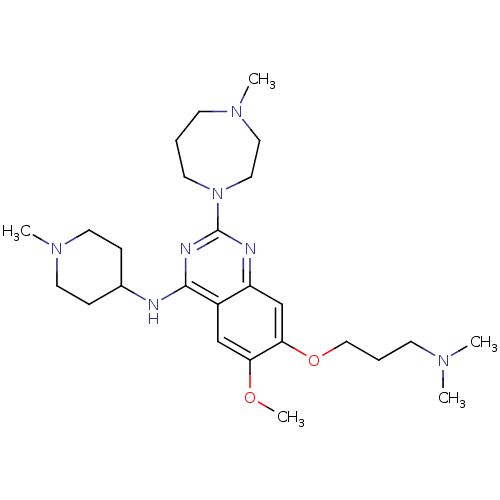
(7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...)Show SMILES COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 Show InChI InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using histone H3 (1 to 25 residues) as substrate preincubated for 2 mins followed by substrate addition measured f... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of G9a (unknown origin) by Morrison plot analysis in presence of histone H3 (1 to 25 residues) |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484345
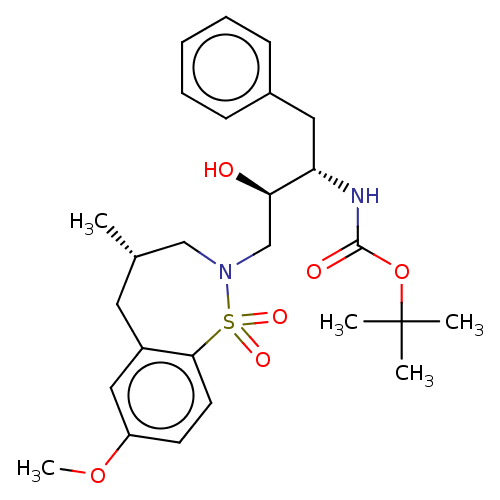
(CHEMBL1835926)Show SMILES COc1ccc2c(C[C@H](C)CN(C[C@@H](O)[C@H](Cc3ccccc3)NC(=O)OC(C)(C)C)S2(=O)=O)c1 |r| Show InChI InChI=1S/C26H36N2O6S/c1-18-13-20-15-21(33-5)11-12-24(20)35(31,32)28(16-18)17-23(29)22(14-19-9-7-6-8-10-19)27-25(30)34-26(2,3)4/h6-12,15,18,22-23,29H,13-14,16-17H2,1-5H3,(H,27,30)/t18-,22-,23+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484351
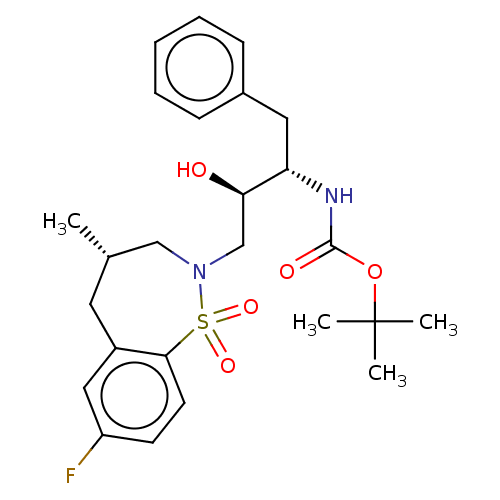
(CHEMBL1835923)Show SMILES C[C@@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)S(=O)(=O)c2ccc(F)cc2C1 |r| Show InChI InChI=1S/C25H33FN2O5S/c1-17-12-19-14-20(26)10-11-23(19)34(31,32)28(15-17)16-22(29)21(13-18-8-6-5-7-9-18)27-24(30)33-25(2,3)4/h5-11,14,17,21-22,29H,12-13,15-16H2,1-4H3,(H,27,30)/t17-,21-,22+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484348
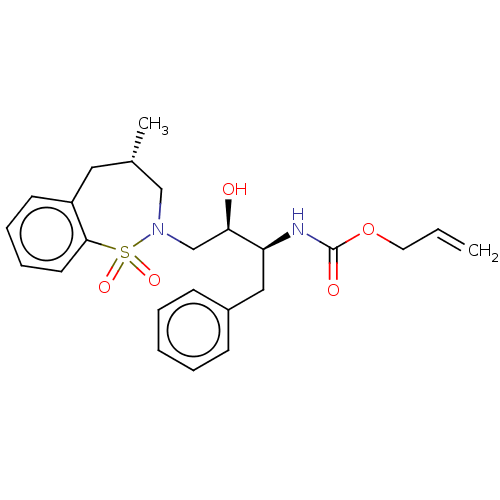
(CHEMBL1835837)Show SMILES C[C@@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)OCC=C)S(=O)(=O)c2ccccc2C1 |r| Show InChI InChI=1S/C24H30N2O5S/c1-3-13-31-24(28)25-21(15-19-9-5-4-6-10-19)22(27)17-26-16-18(2)14-20-11-7-8-12-23(20)32(26,29)30/h3-12,18,21-22,27H,1,13-17H2,2H3,(H,25,28)/t18-,21-,22+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484344
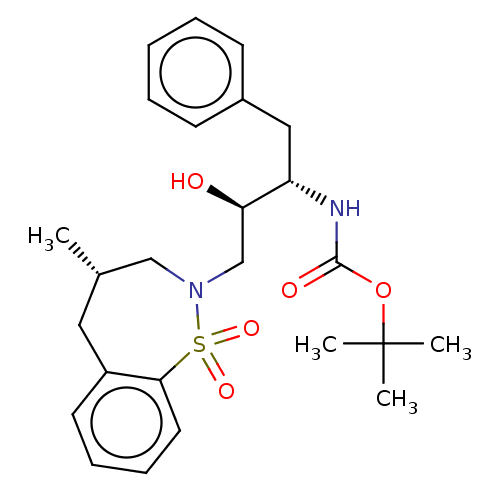
(CHEMBL1835928)Show SMILES C[C@@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)S(=O)(=O)c2ccccc2C1 |r| Show InChI InChI=1S/C25H34N2O5S/c1-18-14-20-12-8-9-13-23(20)33(30,31)27(16-18)17-22(28)21(15-19-10-6-5-7-11-19)26-24(29)32-25(2,3)4/h5-13,18,21-22,28H,14-17H2,1-4H3,(H,26,29)/t18-,21-,22+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484349
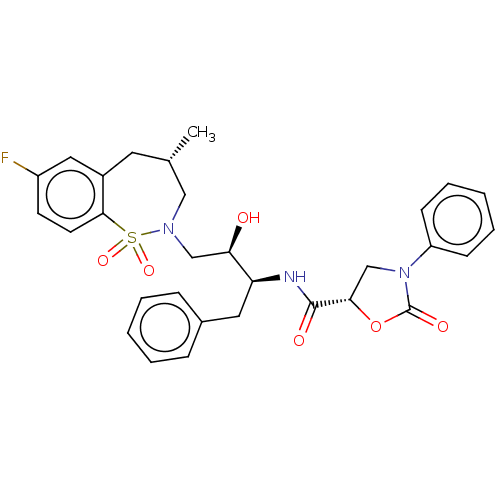
(CHEMBL1835931)Show SMILES C[C@@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CN(C(=O)O2)c2ccccc2)S(=O)(=O)c2ccc(F)cc2C1 |r| Show InChI InChI=1S/C30H32FN3O6S/c1-20-14-22-16-23(31)12-13-28(22)41(38,39)33(17-20)18-26(35)25(15-21-8-4-2-5-9-21)32-29(36)27-19-34(30(37)40-27)24-10-6-3-7-11-24/h2-13,16,20,25-27,35H,14-15,17-19H2,1H3,(H,32,36)/t20-,25-,26+,27-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484347
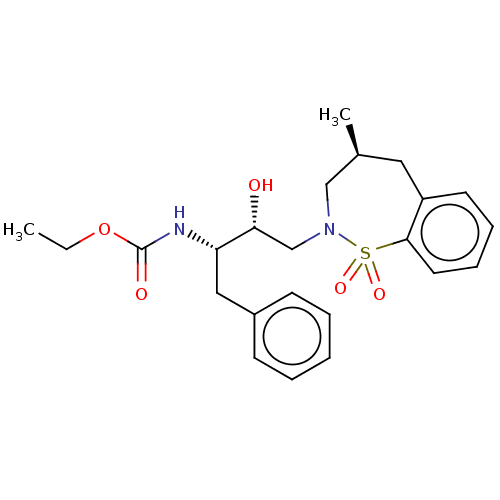
(CHEMBL1835921)Show SMILES CCOC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@@H](C)Cc2ccccc2S1(=O)=O |r| Show InChI InChI=1S/C23H30N2O5S/c1-3-30-23(27)24-20(14-18-9-5-4-6-10-18)21(26)16-25-15-17(2)13-19-11-7-8-12-22(19)31(25,28)29/h4-12,17,20-21,26H,3,13-16H2,1-2H3,(H,24,27)/t17-,20-,21+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484346
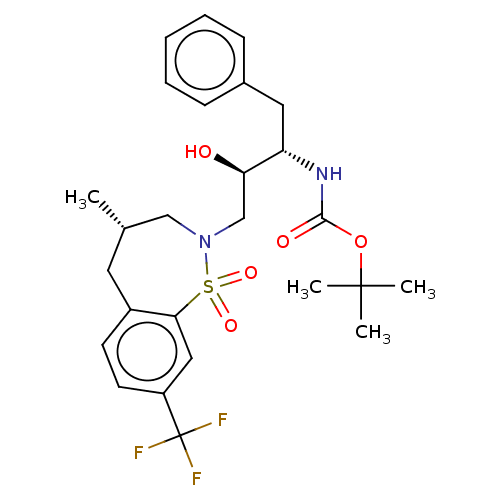
(CHEMBL1835925)Show SMILES C[C@@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)S(=O)(=O)c2cc(ccc2C1)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O5S/c1-17-12-19-10-11-20(26(27,28)29)14-23(19)37(34,35)31(15-17)16-22(32)21(13-18-8-6-5-7-9-18)30-24(33)36-25(2,3)4/h5-11,14,17,21-22,32H,12-13,15-16H2,1-4H3,(H,30,33)/t17-,21-,22+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484353
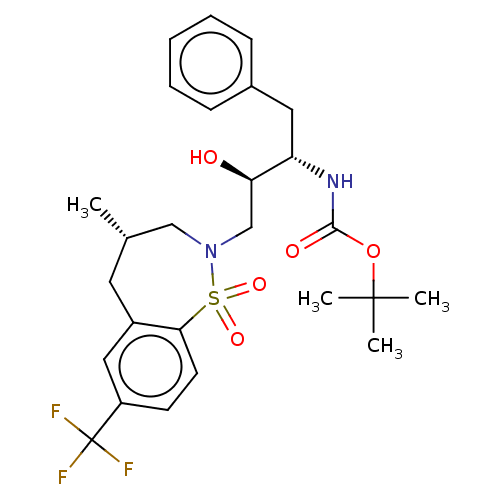
(CHEMBL1835924)Show SMILES C[C@@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)S(=O)(=O)c2ccc(cc2C1)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O5S/c1-17-12-19-14-20(26(27,28)29)10-11-23(19)37(34,35)31(15-17)16-22(32)21(13-18-8-6-5-7-9-18)30-24(33)36-25(2,3)4/h5-11,14,17,21-22,32H,12-13,15-16H2,1-4H3,(H,30,33)/t17-,21-,22+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484350

(CHEMBL1835922)Show SMILES C[C@@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)Oc2ccccc2)S(=O)(=O)c2ccccc2C1 |r| Show InChI InChI=1S/C27H30N2O5S/c1-20-16-22-12-8-9-15-26(22)35(32,33)29(18-20)19-25(30)24(17-21-10-4-2-5-11-21)28-27(31)34-23-13-6-3-7-14-23/h2-15,20,24-25,30H,16-19H2,1H3,(H,28,31)/t20-,24-,25+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484352
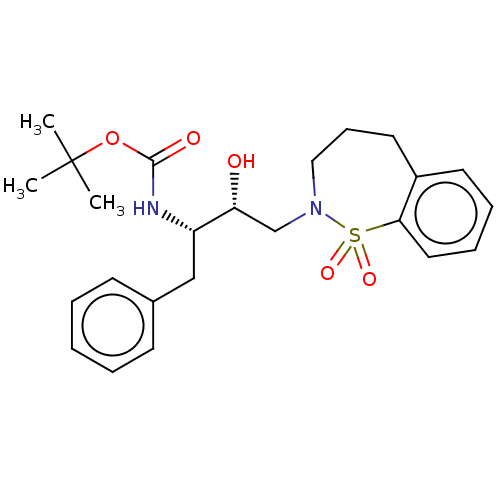
(CHEMBL1835929)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CCCc2ccccc2S1(=O)=O |r| Show InChI InChI=1S/C24H32N2O5S/c1-24(2,3)31-23(28)25-20(16-18-10-5-4-6-11-18)21(27)17-26-15-9-13-19-12-7-8-14-22(19)32(26,29)30/h4-8,10-12,14,20-21,27H,9,13,15-17H2,1-3H3,(H,25,28)/t20-,21+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484354
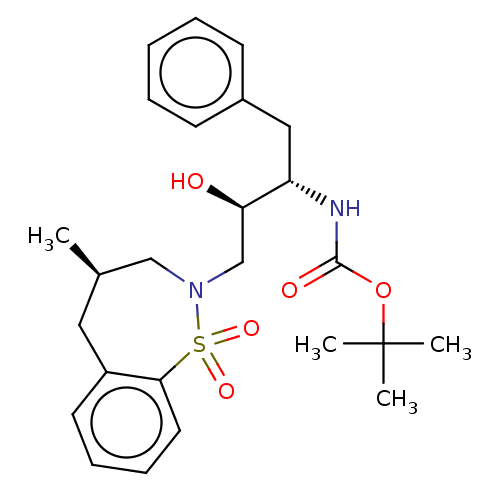
(CHEMBL1835927)Show SMILES C[C@H]1CN(C[C@@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)S(=O)(=O)c2ccccc2C1 |r| Show InChI InChI=1S/C25H34N2O5S/c1-18-14-20-12-8-9-13-23(20)33(30,31)27(16-18)17-22(28)21(15-19-10-6-5-7-11-19)26-24(29)32-25(2,3)4/h5-13,18,21-22,28H,14-17H2,1-4H3,(H,26,29)/t18-,21+,22-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stevens Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease activity after 60 mins by HPLC analysis |
J Med Chem 54: 7176-83 (2011)
Article DOI: 10.1021/jm200778q
BindingDB Entry DOI: 10.7270/Q2VD7291 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50361800
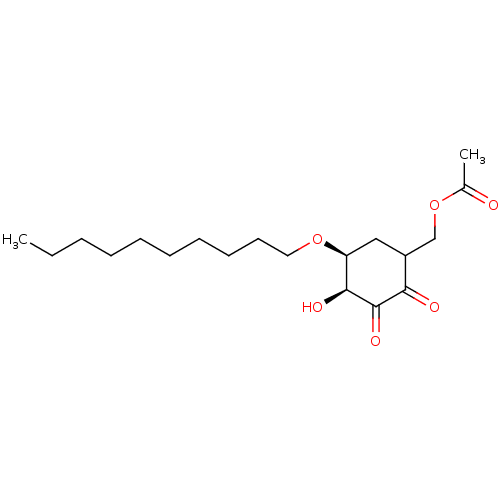
(CHEMBL1938641)Show SMILES CCCCCCCCCCO[C@H]1CC(COC(C)=O)C(=O)C(=O)[C@H]1O |r| Show InChI InChI=1S/C19H32O6/c1-3-4-5-6-7-8-9-10-11-24-16-12-15(13-25-14(2)20)17(21)19(23)18(16)22/h15-16,18,22H,3-13H2,1-2H3/t15?,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human GSTM1 using GSH as substrate by Lineweaver-Burk plot analysis |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50361800

(CHEMBL1938641)Show SMILES CCCCCCCCCCO[C@H]1CC(COC(C)=O)C(=O)C(=O)[C@H]1O |r| Show InChI InChI=1S/C19H32O6/c1-3-4-5-6-7-8-9-10-11-24-16-12-15(13-25-14(2)20)17(21)19(23)18(16)22/h15-16,18,22H,3-13H2,1-2H3/t15?,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human GSTM1 using CDNB as substrate by Lineweaver-Burk plot analysis |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50442103
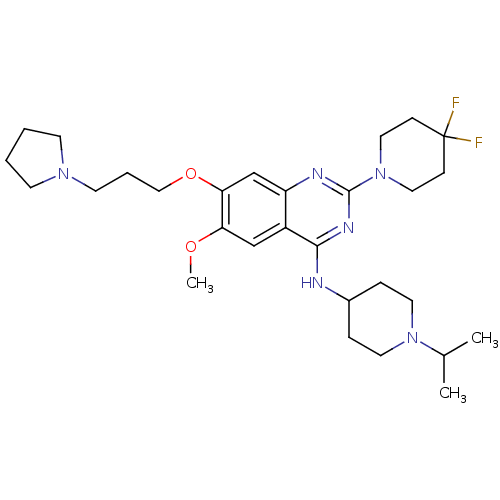
(CHEMBL2441082)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)N1CCC(F)(F)CC1 Show InChI InChI=1S/C29H44F2N6O2/c1-21(2)36-14-7-22(8-15-36)32-27-23-19-25(38-3)26(39-18-6-13-35-11-4-5-12-35)20-24(23)33-28(34-27)37-16-9-29(30,31)10-17-37/h19-22H,4-18H2,1-3H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50446376
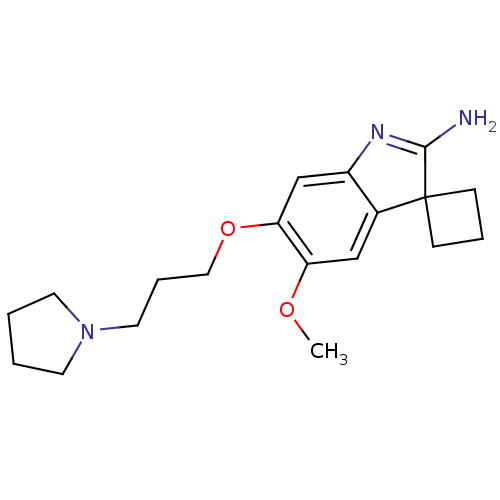
(CHEMBL3109630)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(N)C21CCC1 |t:19| Show InChI InChI=1S/C19H27N3O2/c1-23-16-12-14-15(21-18(20)19(14)6-4-7-19)13-17(16)24-11-5-10-22-8-2-3-9-22/h12-13H,2-11H2,1H3,(H2,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300041

(7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...)Show SMILES COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 Show InChI InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using histone H3 (1 to 25 residues) as substrate preincubated for 2 mins followed by substrate addition measured f... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <15 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using histone H3 (1 to 25 residues) as substrate preincubated for 2 mins followed by substrate addition measured f... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50523996
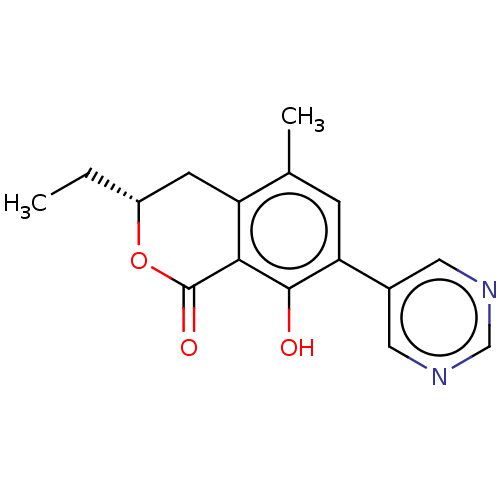
(CHEMBL4549753)Show SMILES CC[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1cncnc1 |r| Show InChI InChI=1S/C16H16N2O3/c1-3-11-5-12-9(2)4-13(10-6-17-8-18-7-10)15(19)14(12)16(20)21-11/h4,6-8,11,19H,3,5H2,1-2H3/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50022279
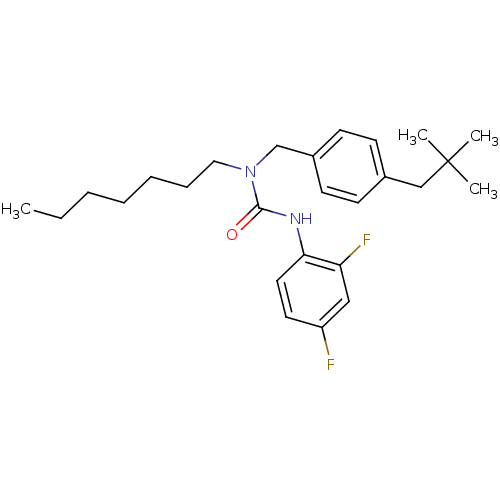
(3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...)Show SMILES CCCCCCCN(Cc1ccc(CC(C)(C)C)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H36F2N2O/c1-5-6-7-8-9-16-30(25(31)29-24-15-14-22(27)17-23(24)28)19-21-12-10-20(11-13-21)18-26(2,3)4/h10-15,17H,5-9,16,18-19H2,1-4H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of smooth muscle cell ACAT activity for cells stimulated by cationized LDL. |
J Med Chem 29: 1131-3 (1987)
BindingDB Entry DOI: 10.7270/Q2V69HKP |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50523988
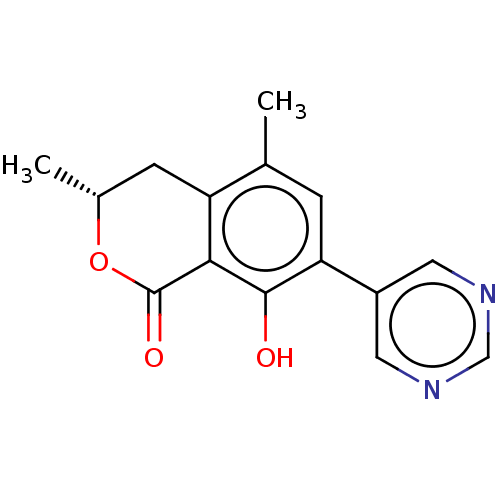
(CHEMBL4448210)Show SMILES C[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1cncnc1 |r| Show InChI InChI=1S/C15H14N2O3/c1-8-3-12(10-5-16-7-17-6-10)14(18)13-11(8)4-9(2)20-15(13)19/h3,5-7,9,18H,4H2,1-2H3/t9-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50524023
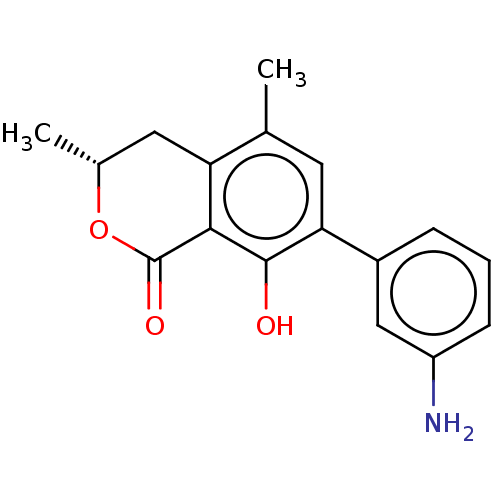
(CHEMBL4450374)Show SMILES C[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1cccc(N)c1 |r| Show InChI InChI=1S/C17H17NO3/c1-9-6-14(11-4-3-5-12(18)8-11)16(19)15-13(9)7-10(2)21-17(15)20/h3-6,8,10,19H,7,18H2,1-2H3/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50523998
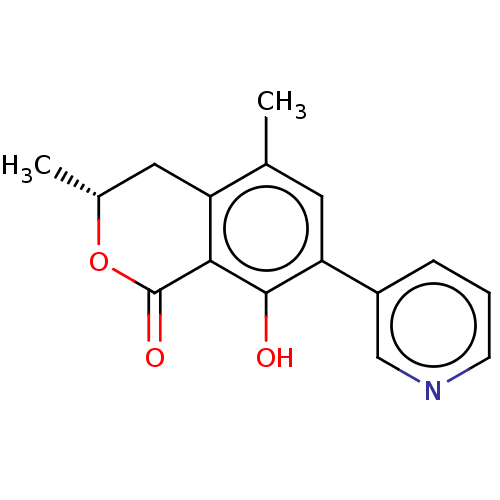
(CHEMBL4539671)Show SMILES C[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1cccnc1 |r| Show InChI InChI=1S/C16H15NO3/c1-9-6-13(11-4-3-5-17-8-11)15(18)14-12(9)7-10(2)20-16(14)19/h3-6,8,10,18H,7H2,1-2H3/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594333
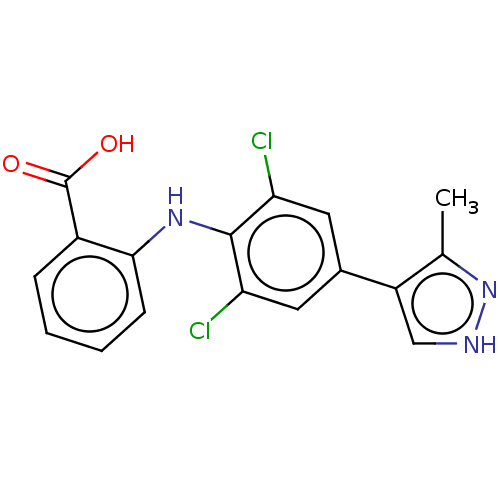
(CHEMBL5174419)Show SMILES Cc1n[nH]cc1-c1cc(Cl)c(Nc2ccccc2C(O)=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50523995
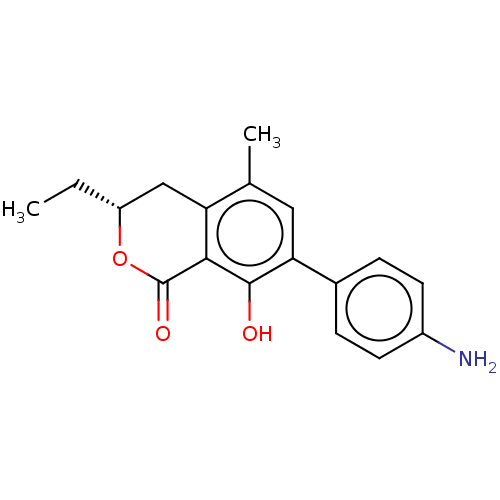
(CHEMBL4526304)Show SMILES CC[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1ccc(N)cc1 |r| Show InChI InChI=1S/C18H19NO3/c1-3-13-9-14-10(2)8-15(11-4-6-12(19)7-5-11)17(20)16(14)18(21)22-13/h4-8,13,20H,3,9,19H2,1-2H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM589235
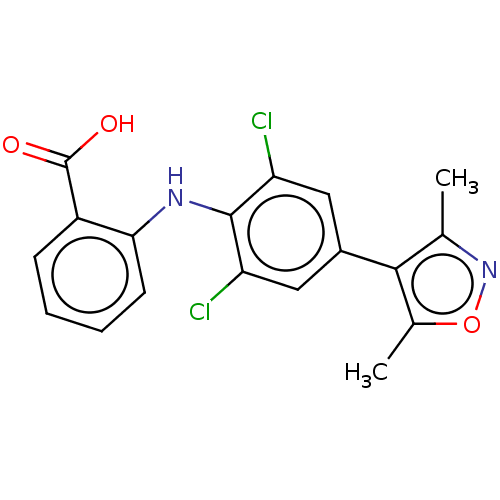
(US11555009, Compound 15 | US11555009, Compound 29)Show SMILES Cc1noc(C)c1-c1cc(Cl)c(Nc2ccccc2C(O)=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM589236
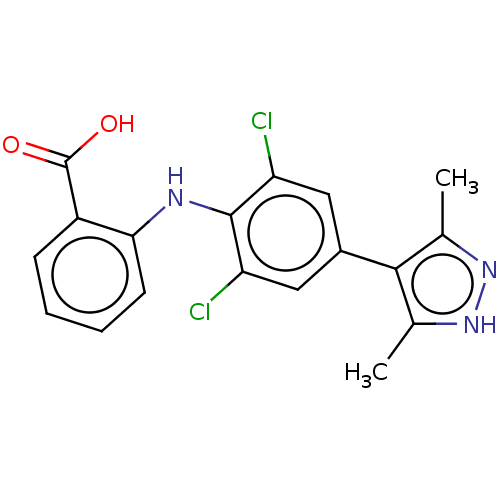
(US11555009, Compound 17)Show SMILES Cc1n[nH]c(C)c1-c1cc(Cl)c(Nc2ccccc2C(O)=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594336
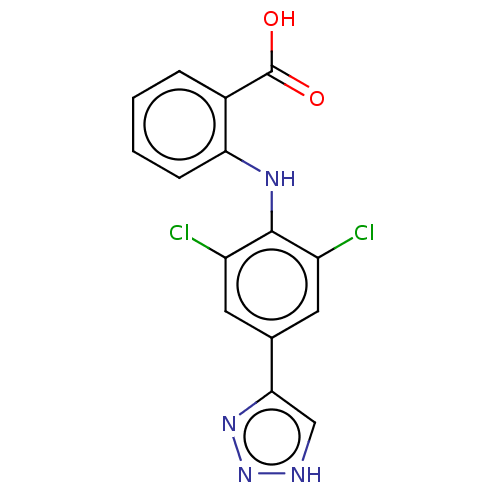
(CHEMBL5176527) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using biotinylated-histone H3 (1 to 21 residues)/S-adenosyl-methionine as substrate/methyl donor after 3 hrs by Al... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594337
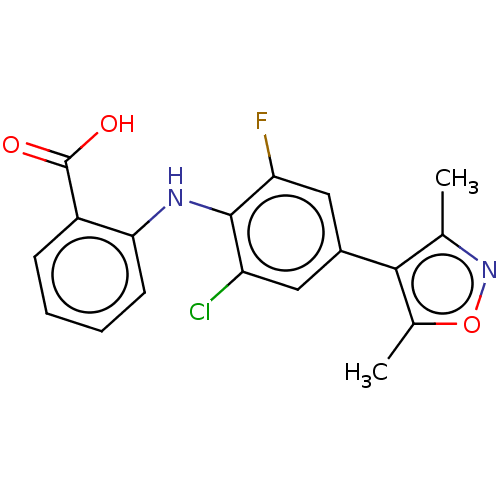
(CHEMBL5176730)Show SMILES Cc1noc(C)c1-c1cc(F)c(Nc2ccccc2C(O)=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50022279

(3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...)Show SMILES CCCCCCCN(Cc1ccc(CC(C)(C)C)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H36F2N2O/c1-5-6-7-8-9-16-30(25(31)29-24-15-14-22(27)17-23(24)28)19-21-12-10-20(11-13-21)18-26(2,3)4/h10-15,17H,5-9,16,18-19H2,1-4H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in Liver microsomes |
J Med Chem 29: 1131-3 (1987)
BindingDB Entry DOI: 10.7270/Q2V69HKP |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50523999
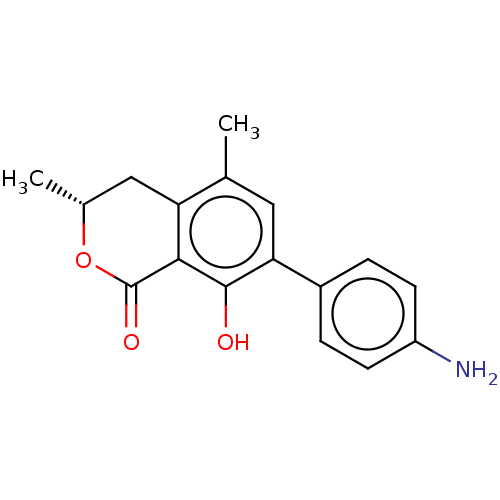
(CHEMBL4593138)Show SMILES C[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1ccc(N)cc1 |r| Show InChI InChI=1S/C17H17NO3/c1-9-7-14(11-3-5-12(18)6-4-11)16(19)15-13(9)8-10(2)21-17(15)20/h3-7,10,19H,8,18H2,1-2H3/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50022279

(3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...)Show SMILES CCCCCCCN(Cc1ccc(CC(C)(C)C)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H36F2N2O/c1-5-6-7-8-9-16-30(25(31)29-24-15-14-22(27)17-23(24)28)19-21-12-10-20(11-13-21)18-26(2,3)4/h10-15,17H,5-9,16,18-19H2,1-4H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in intestinal microsomes |
J Med Chem 29: 1131-3 (1987)
BindingDB Entry DOI: 10.7270/Q2V69HKP |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594341
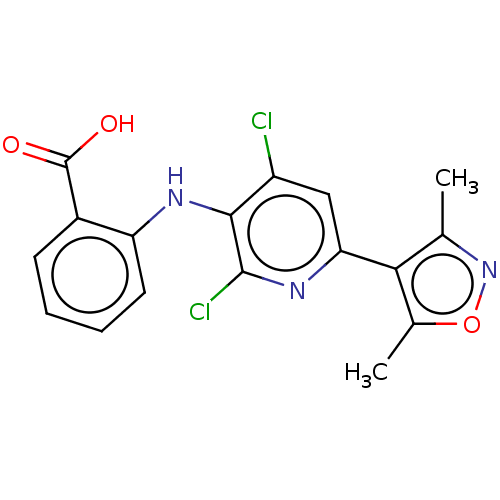
(CHEMBL5173835)Show SMILES Cc1noc(C)c1-c1cc(Cl)c(Nc2ccccc2C(O)=O)c(Cl)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594335
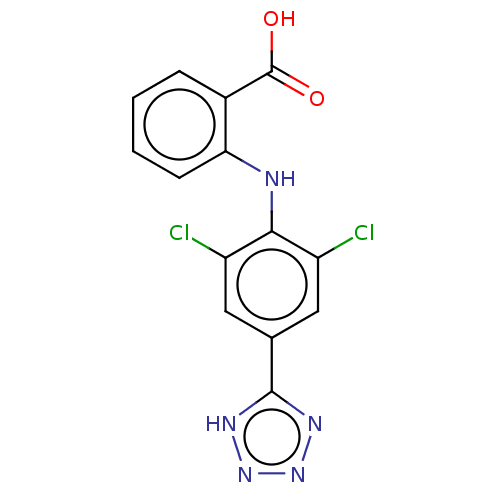
(CHEMBL4436398) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594340
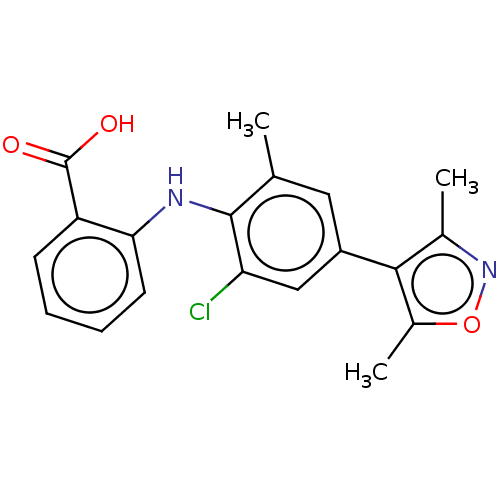
(CHEMBL5171501)Show SMILES Cc1noc(C)c1-c1cc(C)c(Nc2ccccc2C(O)=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50524001
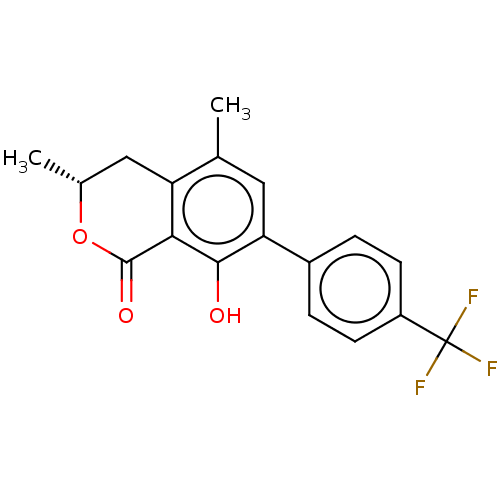
(CHEMBL4483744)Show SMILES C[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C18H15F3O3/c1-9-7-14(11-3-5-12(6-4-11)18(19,20)21)16(22)15-13(9)8-10(2)24-17(15)23/h3-7,10,22H,8H2,1-2H3/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50523984
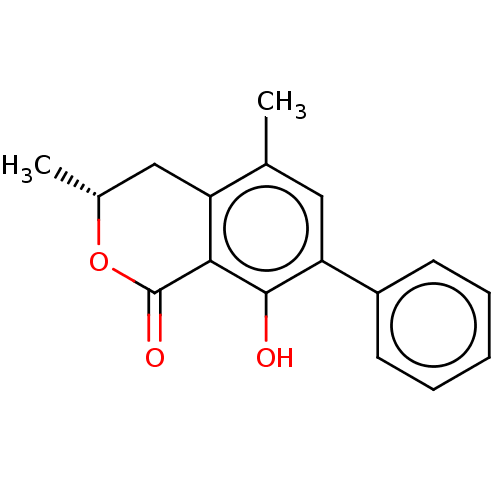
(CHEMBL4547510)Show SMILES C[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1ccccc1 |r| Show InChI InChI=1S/C17H16O3/c1-10-8-14(12-6-4-3-5-7-12)16(18)15-13(10)9-11(2)20-17(15)19/h3-8,11,18H,9H2,1-2H3/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A/B
(Mus musculus) | BDBM50523994
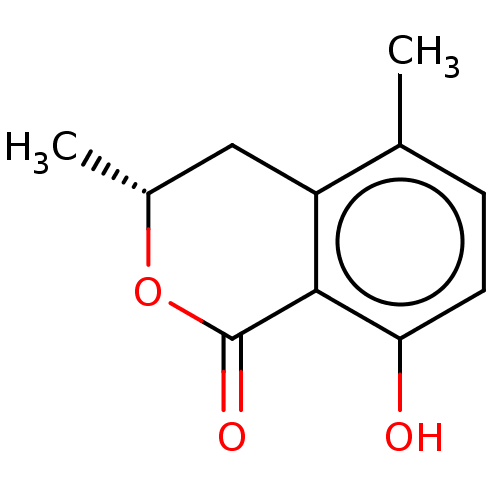
(CHEMBL461985)Show InChI InChI=1S/C11H12O3/c1-6-3-4-9(12)10-8(6)5-7(2)14-11(10)13/h3-4,7,12H,5H2,1-2H3/t7-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain monoamine oxidase |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50022279

(3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...)Show SMILES CCCCCCCN(Cc1ccc(CC(C)(C)C)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C26H36F2N2O/c1-5-6-7-8-9-16-30(25(31)29-24-15-14-22(27)17-23(24)28)19-21-12-10-20(11-13-21)18-26(2,3)4/h10-15,17H,5-9,16,18-19H2,1-4H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in Aorta (homogenate Low-density lipoproteins (LDL) |
J Med Chem 29: 1131-3 (1987)
BindingDB Entry DOI: 10.7270/Q2V69HKP |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594345
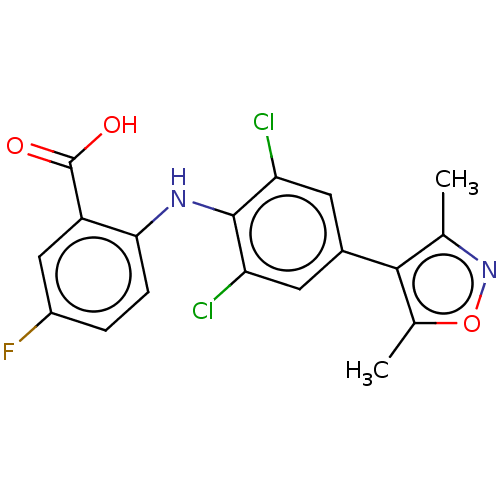
(CHEMBL5190268)Show SMILES Cc1noc(C)c1-c1cc(Cl)c(Nc2ccc(F)cc2C(O)=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50524000
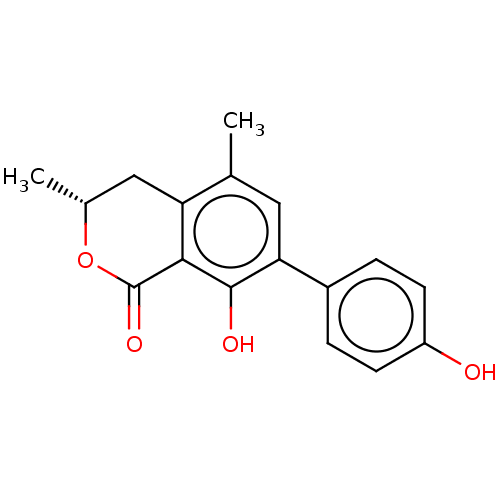
(CHEMBL4581266)Show SMILES C[C@@H]1Cc2c(C)cc(c(O)c2C(=O)O1)-c1ccc(O)cc1 |r| Show InChI InChI=1S/C17H16O4/c1-9-7-14(11-3-5-12(18)6-4-11)16(19)15-13(9)8-10(2)21-17(15)20/h3-7,10,18-19H,8H2,1-2H3/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysis |
Bioorg Med Chem 27: 2027-2040 (2019)
Article DOI: 10.1016/j.bmc.2019.03.060
BindingDB Entry DOI: 10.7270/Q2BG2SD6 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50195457
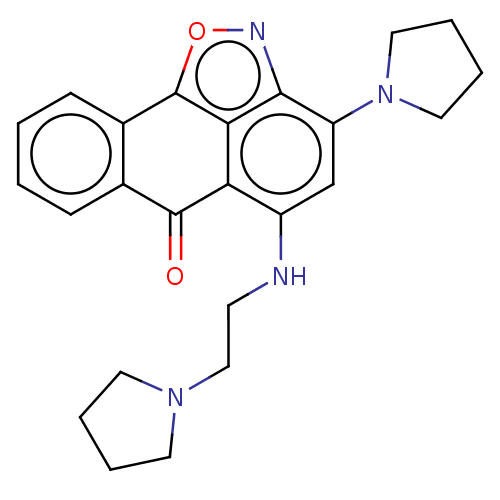
(CHEMBL3943729)Show SMILES O=C1c2ccccc2-c2onc3c(cc(NCCN4CCCC4)c1c23)N1CCCC1 Show InChI InChI=1S/C24H26N4O2/c29-23-16-7-1-2-8-17(16)24-21-20(23)18(25-9-14-27-10-3-4-11-27)15-19(22(21)26-30-24)28-12-5-6-13-28/h1-2,7-8,15,25H,3-6,9-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using biotinylated-histone H3 (1 to 21 residues)/S-adenosyl-methionine as substrate/methyl donor after 3 hrs by Al... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594339
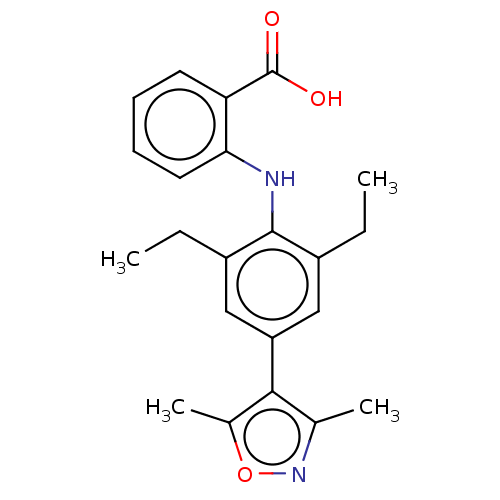
(CHEMBL5190139)Show SMILES CCc1cc(cc(CC)c1Nc1ccccc1C(O)=O)-c1c(C)noc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM589231
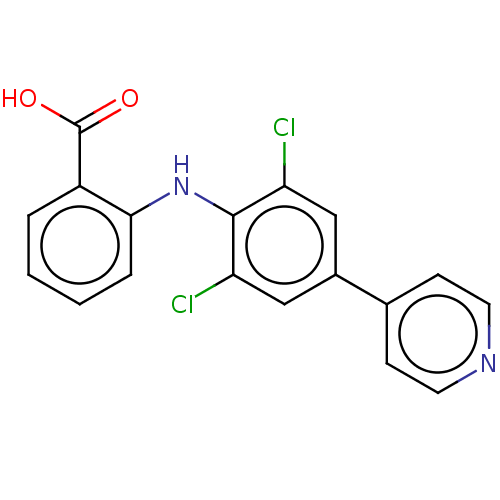
(US11555009, Compound 5) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594342
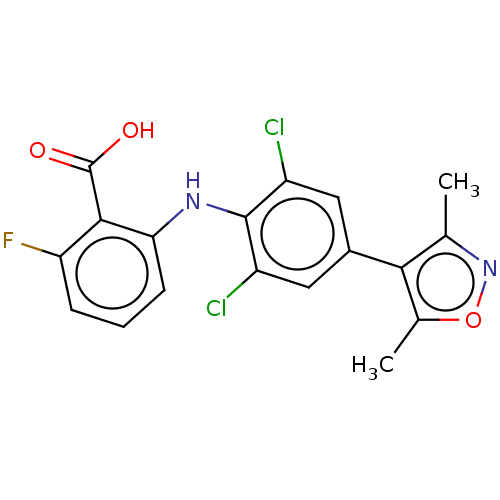
(CHEMBL5175318)Show SMILES Cc1noc(C)c1-c1cc(Cl)c(Nc2cccc(F)c2C(O)=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50594334
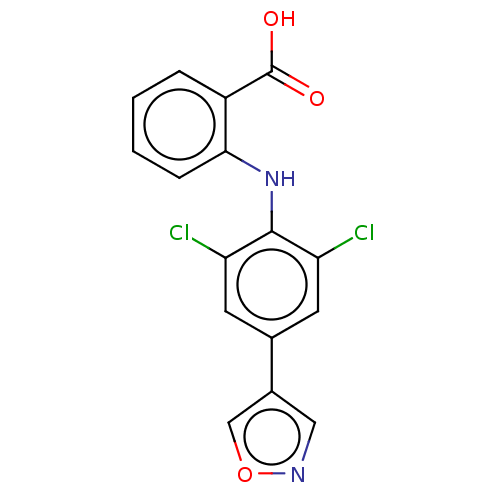
(CHEMBL5206682) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00848
BindingDB Entry DOI: 10.7270/Q2959NJK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50195466
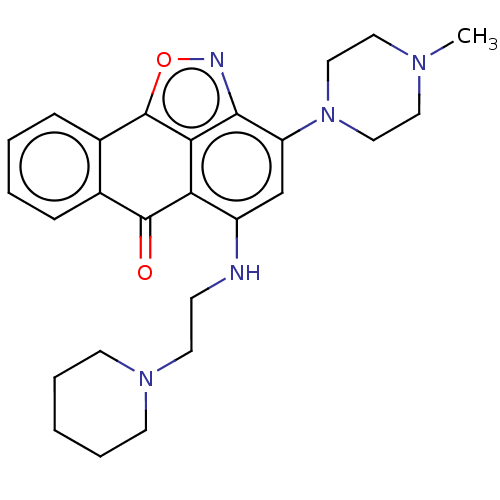
(CHEMBL3948437)Show SMILES CN1CCN(CC1)c1cc(NCCN2CCCCC2)c2C(=O)c3ccccc3-c3onc1c23 Show InChI InChI=1S/C26H31N5O2/c1-29-13-15-31(16-14-29)21-17-20(27-9-12-30-10-5-2-6-11-30)22-23-24(21)28-33-26(23)19-8-4-3-7-18(19)25(22)32/h3-4,7-8,17,27H,2,5-6,9-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using biotinylated-histone H3 (1 to 21 residues)/S-adenosyl-methionine as substrate/methyl donor after 3 hrs by Al... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data