

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079508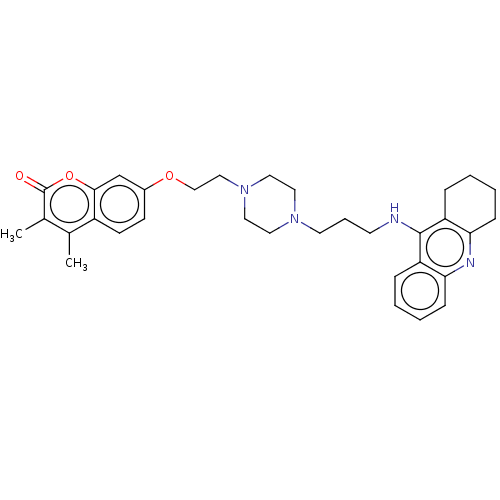 (CHEMBL3417300) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE by Lineweaver-Burk plot analysis | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50282506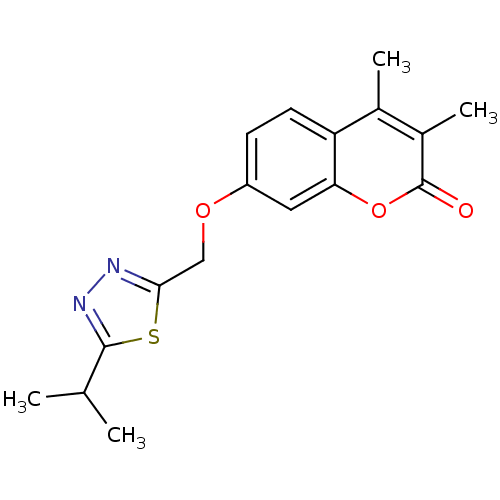 (7-(5-Isopropyl-[1,3,4]thiadiazol-2-ylmethoxy)-3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MAO-B (unknown origin) | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409078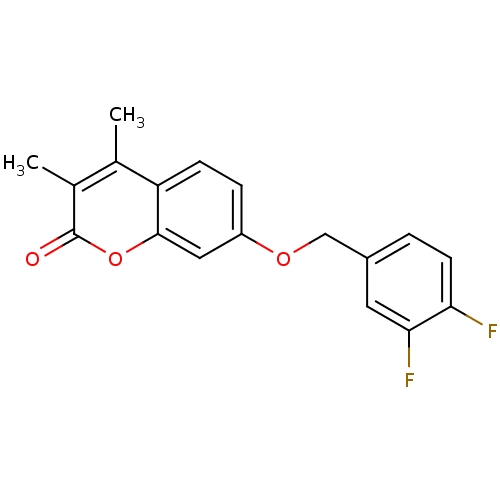 (CHEMBL325761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199097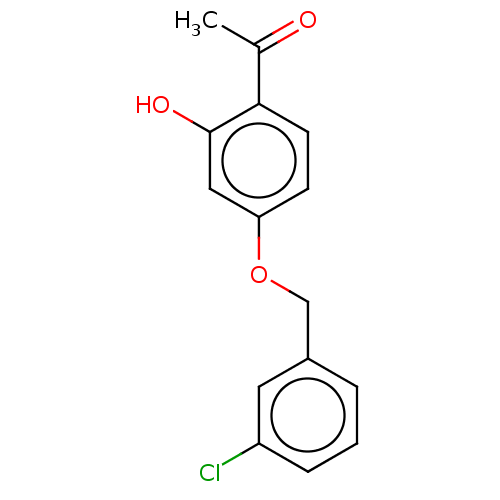 (CHEMBL3911291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production incubated for 20 mins by fl... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199101 (CHEMBL3920226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production incubated for 20 mins by fl... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50409097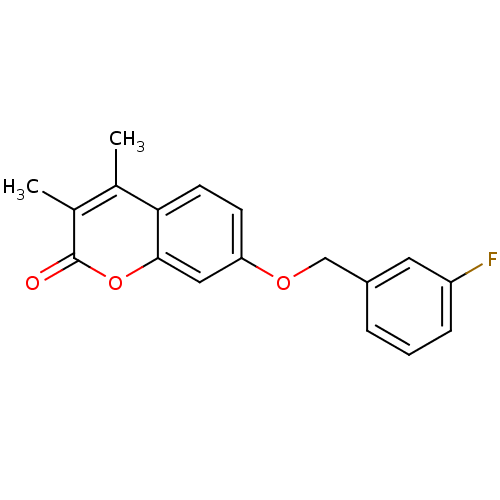 (CHEMBL108697) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50282510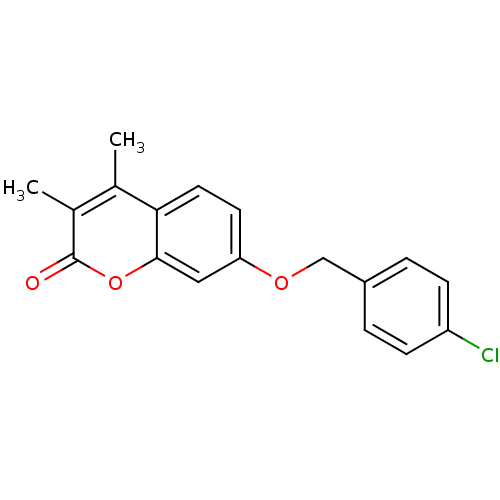 (7-(4-Chloro-benzyloxy)-3,4-dimethyl-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199094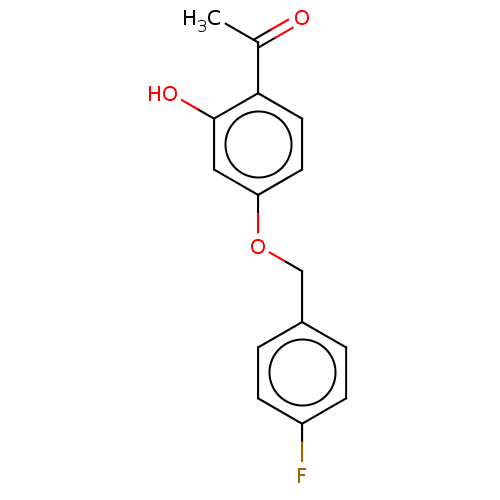 (CHEMBL3948027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production incubated for 20 mins by fl... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50409078 (CHEMBL325761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50409097 (CHEMBL108697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073116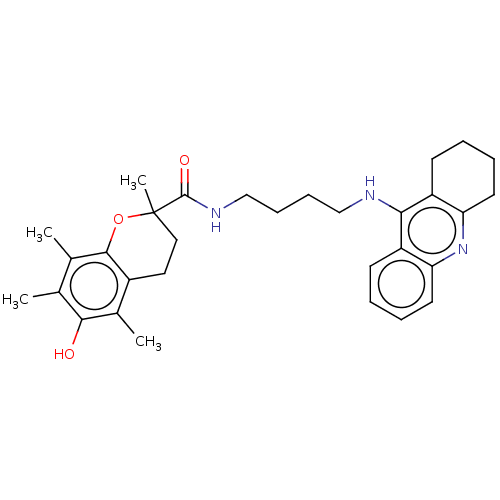 (CHEMBL3410952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199103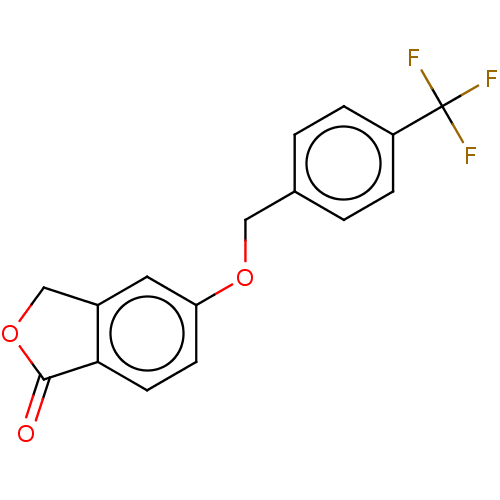 (CHEMBL3938837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50282510 (7-(4-Chloro-benzyloxy)-3,4-dimethyl-chromen-2-one ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50017427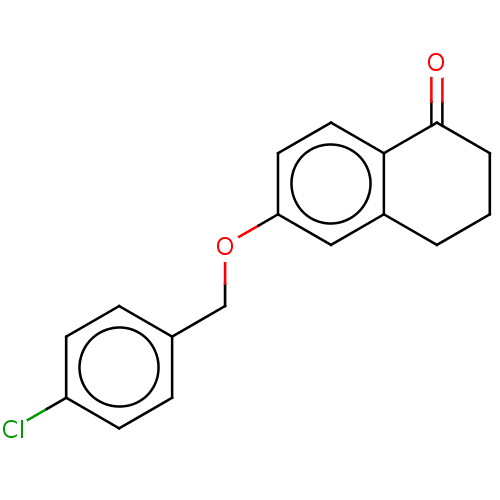 (CHEMBL3288295) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989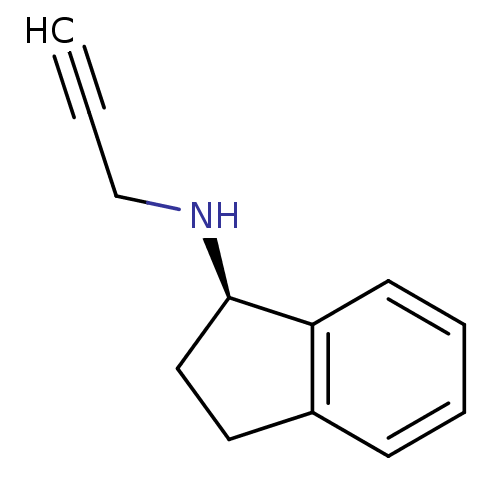 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM92663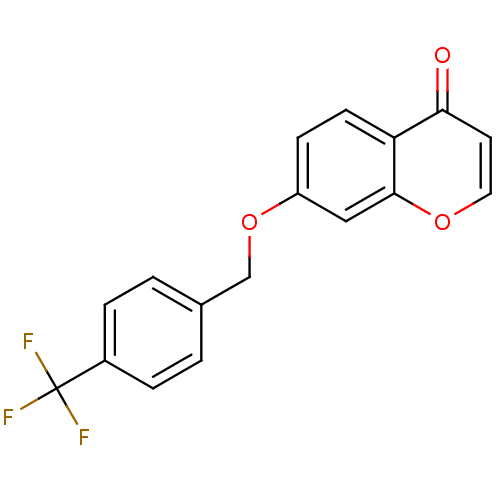 (C7-substituted chromone derivative, 3n) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in insect cell microsomes using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline pr... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199104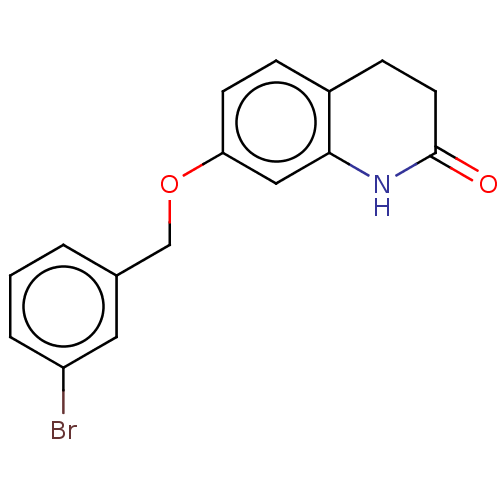 (CHEMBL2430707) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199106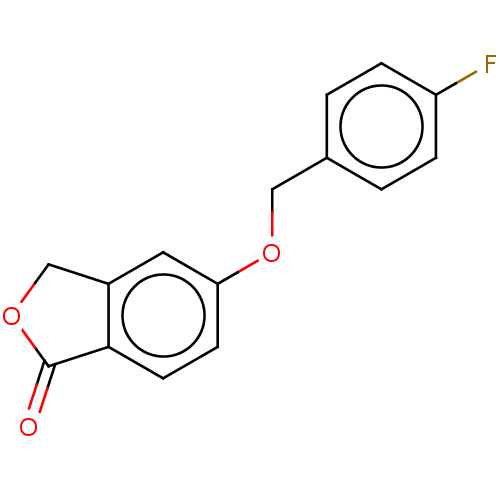 (CHEMBL3929816) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441841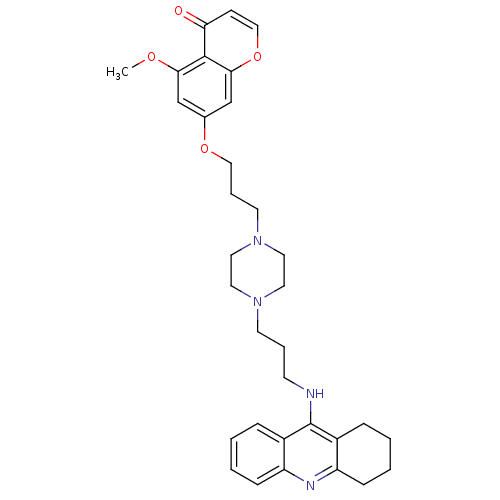 (CHEMBL2436691) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073114 (CHEMBL3410954) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441840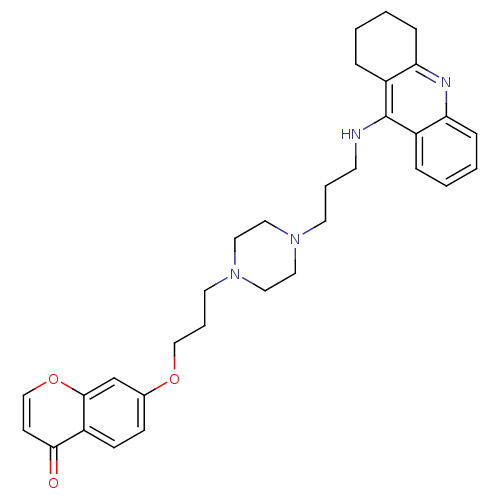 (CHEMBL2436692) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073115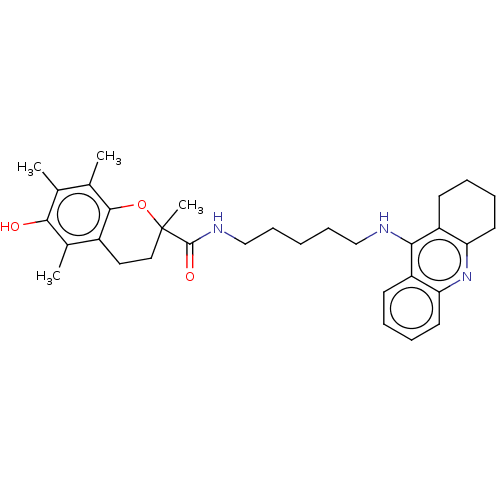 (CHEMBL3410953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199095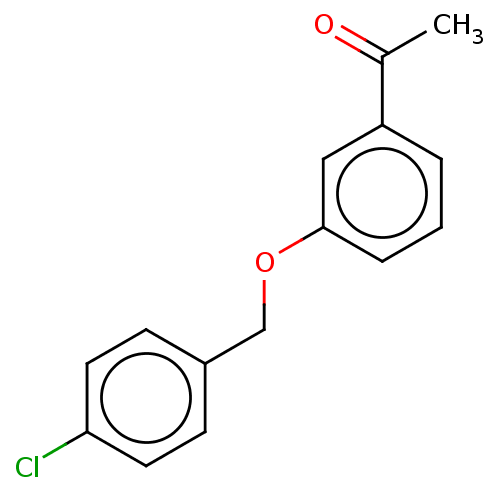 (CHEMBL3771110) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50337524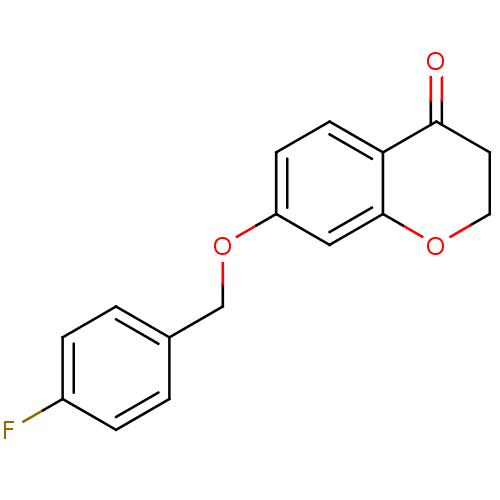 (7-(4-fluorobenzyloxy)chroman-4-one | CHEMBL1682819) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199110 (CHEMBL3741461) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199108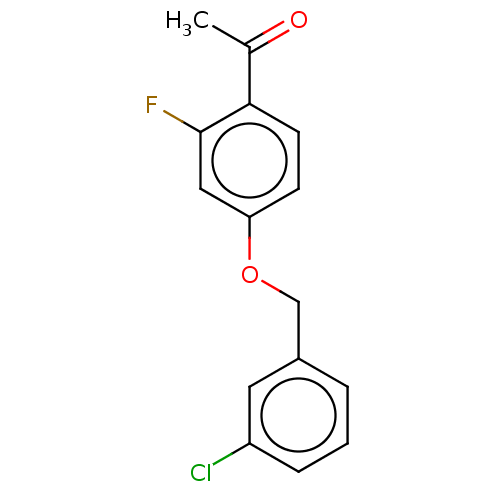 (CHEMBL3919232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50017425 (CHEMBL3288293) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199091 (CHEMBL3910900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199105 (CHEMBL2430706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50441842 (CHEMBL2436690) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition by Ellmans method | Eur J Med Chem 69: 632-46 (2013) Article DOI: 10.1016/j.ejmech.2013.09.024 BindingDB Entry DOI: 10.7270/Q2BP048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199100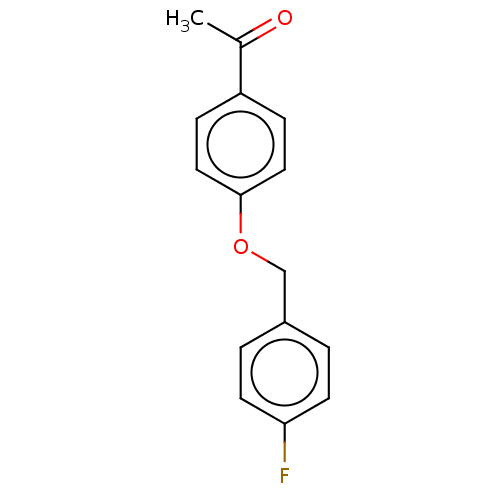 (CHEMBL3770015) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079508 (CHEMBL3417300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180 secs b... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073116 (CHEMBL3410952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079522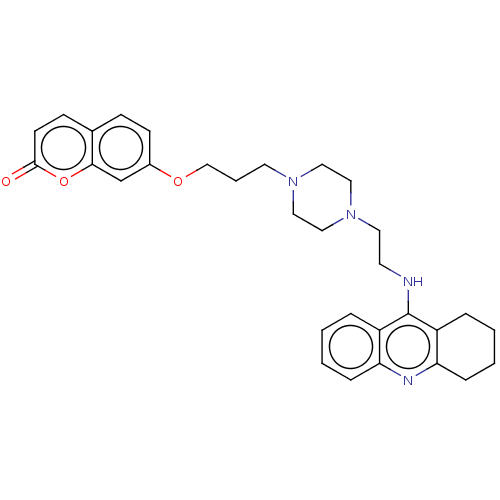 (CHEMBL3417311) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50363844 (CHEMBL1945157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM92653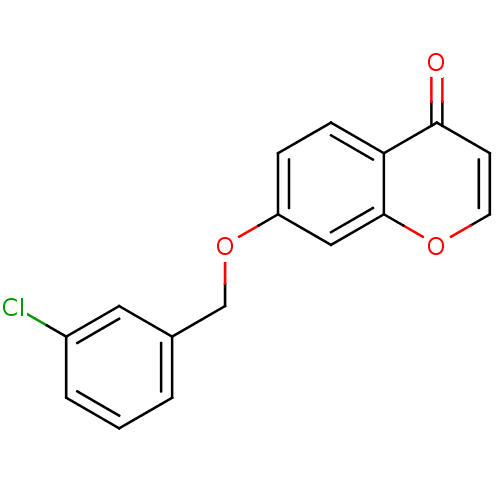 (C7-substituted chromone derivative, 3d) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in insect cell microsomes using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline pr... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079515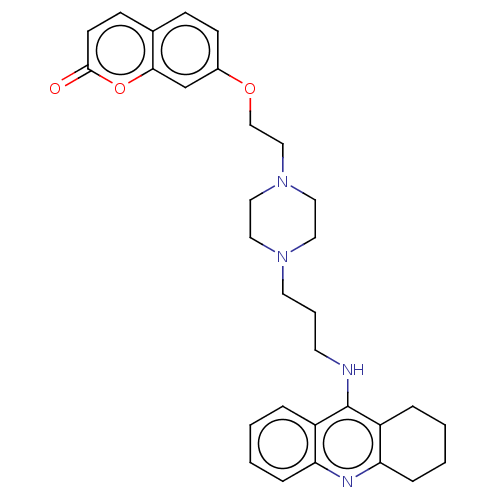 (CHEMBL3417307) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073113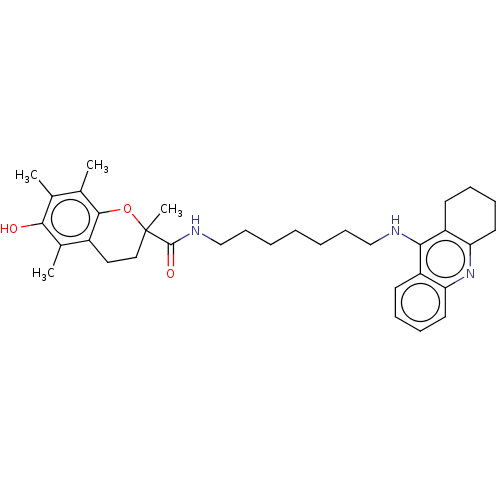 (CHEMBL3410955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079518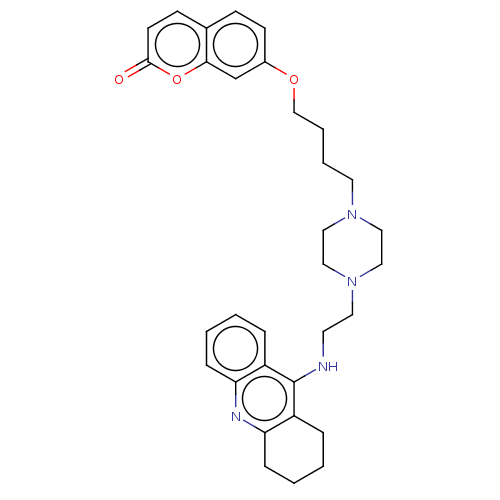 (CHEMBL3417310) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50073114 (CHEMBL3410954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50079513 (CHEMBL3417305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using S-butyrylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to ... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 6 mins before substrate addition by Ellman's method | Eur J Med Chem 93: 42-50 (2015) Article DOI: 10.1016/j.ejmech.2015.01.058 BindingDB Entry DOI: 10.7270/Q2154JRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50079514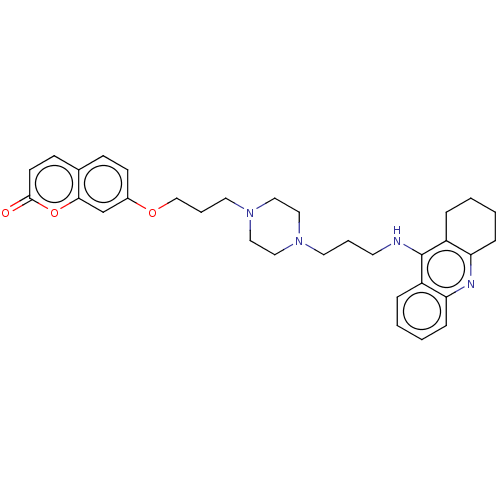 (CHEMBL3417306) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079506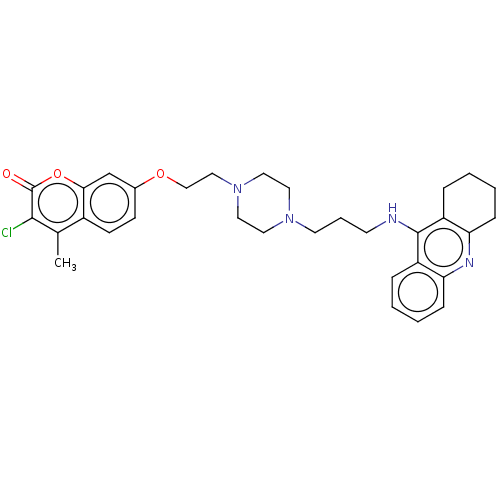 (CHEMBL3417299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins prior to substrate addition measured after 60 to 180 secs b... | Eur J Med Chem 95: 153-65 (2015) Article DOI: 10.1016/j.ejmech.2015.03.040 BindingDB Entry DOI: 10.7270/Q20R9R4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199109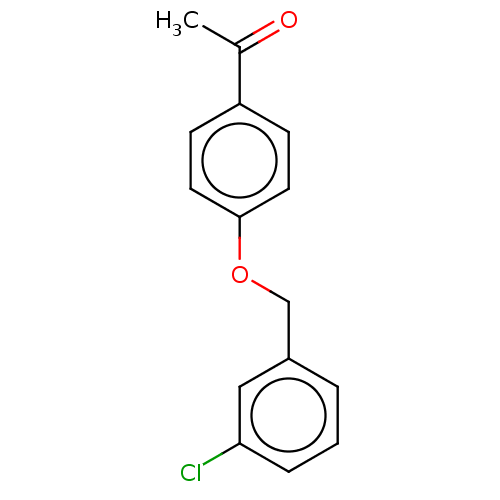 (CHEMBL3771258) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50363840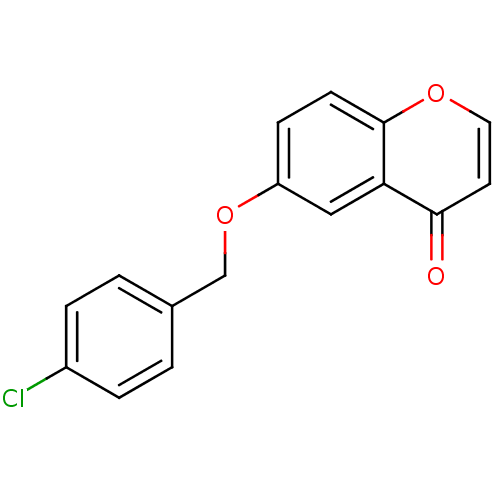 (CHEMBL1945153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50199097 (CHEMBL3911291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 244 total ) | Next | Last >> |