 Found 424 hits with Last Name = 'warne' and Initial = 'b'
Found 424 hits with Last Name = 'warne' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tubulin beta-2B chain
(Bos taurus) | BDBM50005480
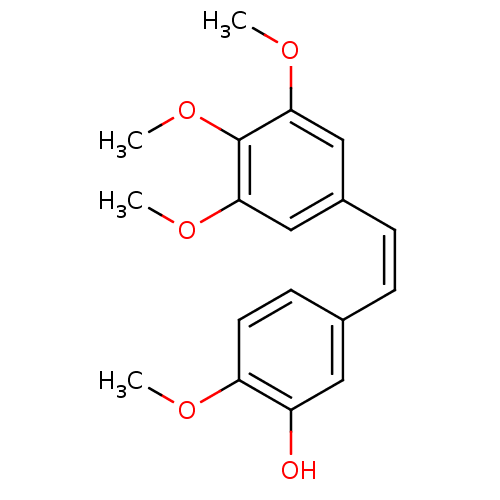
((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50109343
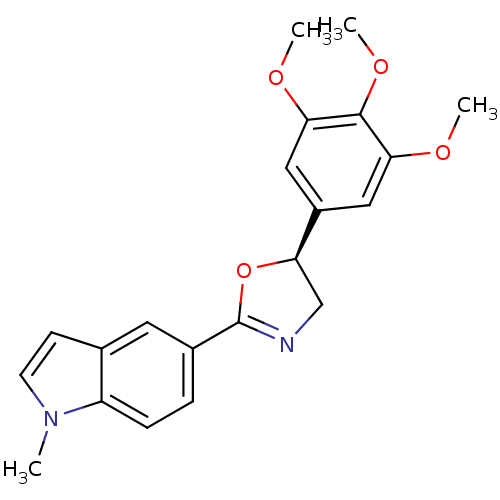
(1-Methyl-5-[(S)-5-(3,4,5-trimethoxy-phenyl)-4,5-di...)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1CN=C(O1)c1ccc2n(C)ccc2c1 |c:15| Show InChI InChI=1S/C21H22N2O4/c1-23-8-7-13-9-14(5-6-16(13)23)21-22-12-19(27-21)15-10-17(24-2)20(26-4)18(11-15)25-3/h5-11,19H,12H2,1-4H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50014846
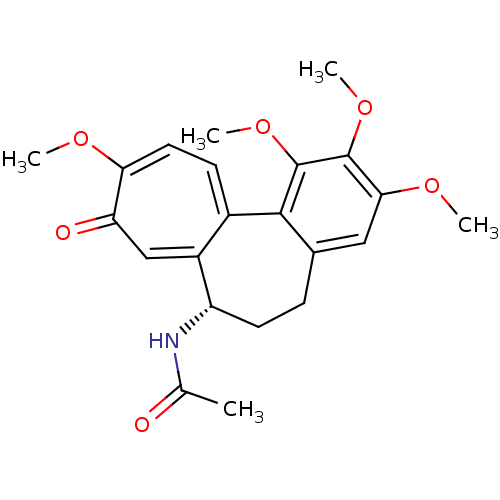
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50109342
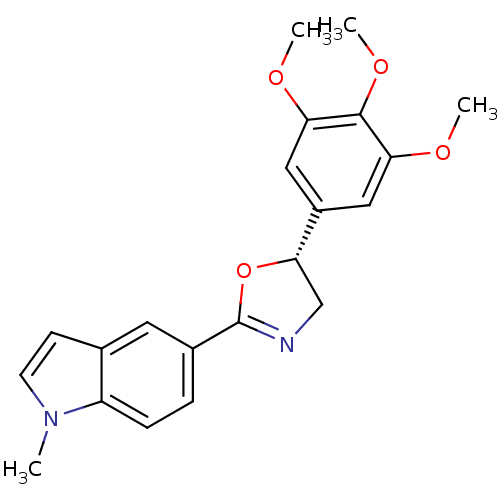
(1-Methyl-5-[(R)-5-(3,4,5-trimethoxy-phenyl)-4,5-di...)Show SMILES COc1cc(cc(OC)c1OC)[C@@H]1CN=C(O1)c1ccc2n(C)ccc2c1 |c:15| Show InChI InChI=1S/C21H22N2O4/c1-23-8-7-13-9-14(5-6-16(13)23)21-22-12-19(27-21)15-10-17(24-2)20(26-4)18(11-15)25-3/h5-11,19H,12H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50012278
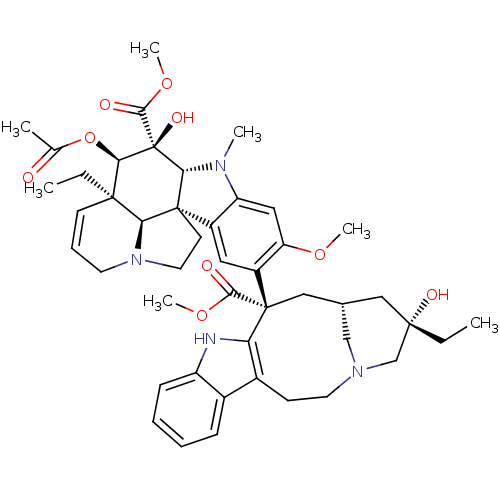
((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...)Show SMILES CC[C@]1(O)C[C@@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC |r,c:48| Show InChI InChI=1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37-,38+,39+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50467097

(CHEMBL4292020)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1c(cnc2[nH]ccc12)-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C24H22F2N4O/c1-29-8-10-30(11-9-29)17-4-2-15(3-5-17)22-18-6-7-27-24(18)28-14-19(22)16-12-20(25)23(31)21(26)13-16/h2-7,12-14,31H,8-11H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem Lett 28: 3197-3201 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.020
BindingDB Entry DOI: 10.7270/Q2F76G78 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500931
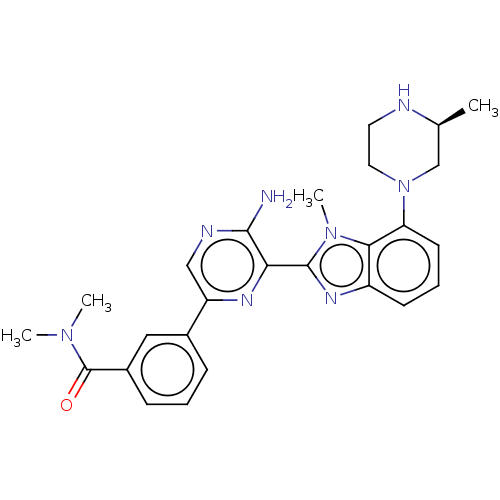
(CHEMBL3797873)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cccc(c3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C26H30N8O/c1-16-15-34(12-11-28-16)21-10-6-9-19-23(21)33(4)25(31-19)22-24(27)29-14-20(30-22)17-7-5-8-18(13-17)26(35)32(2)3/h5-10,13-14,16,28H,11-12,15H2,1-4H3,(H2,27,29)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500936
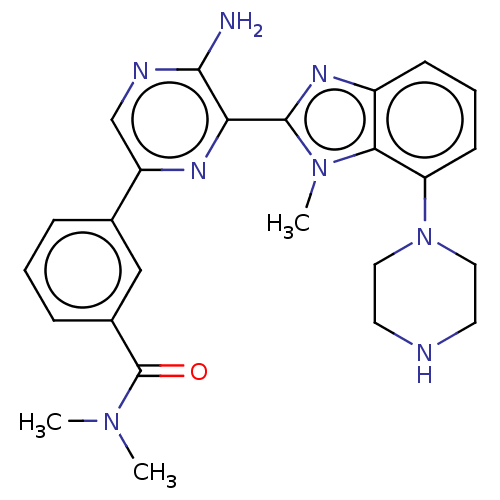
(CHEMBL3798762)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(n1)-c1nc2cccc(N3CCNCC3)c2n1C Show InChI InChI=1S/C25H28N8O/c1-31(2)25(34)17-7-4-6-16(14-17)19-15-28-23(26)21(29-19)24-30-18-8-5-9-20(22(18)32(24)3)33-12-10-27-11-13-33/h4-9,14-15,27H,10-13H2,1-3H3,(H2,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218255
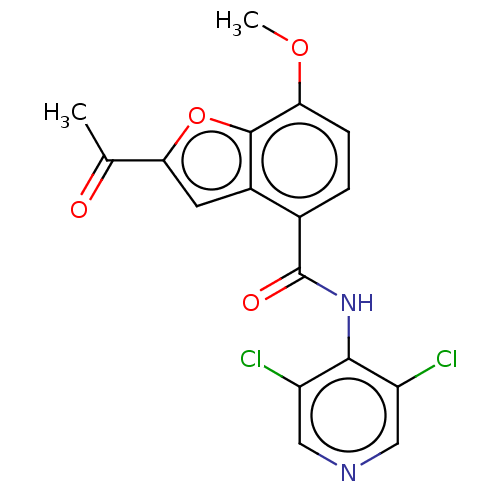
(CHEMBL290100)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(C)=O Show InChI InChI=1S/C17H12Cl2N2O4/c1-8(22)14-5-10-9(3-4-13(24-2)16(10)25-14)17(23)21-15-11(18)6-20-7-12(15)19/h3-7H,1-2H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 (PDE-4) from human U937 cells |
Bioorg Med Chem Lett 12: 1613-5 (2002)
BindingDB Entry DOI: 10.7270/Q2D220S3 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500931

(CHEMBL3797873)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cccc(c3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C26H30N8O/c1-16-15-34(12-11-28-16)21-10-6-9-19-23(21)33(4)25(31-19)22-24(27)29-14-20(30-22)17-7-5-8-18(13-17)26(35)32(2)3/h5-10,13-14,16,28H,11-12,15H2,1-4H3,(H2,27,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218255

(CHEMBL290100)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(C)=O Show InChI InChI=1S/C17H12Cl2N2O4/c1-8(22)14-5-10-9(3-4-13(24-2)16(10)25-14)17(23)21-15-11(18)6-20-7-12(15)19/h3-7H,1-2H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50467103

(CHEMBL4289492)Show SMILES Cc1ccc(cc1)-c1c(cnc2[nH]ccc12)-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C20H14F2N2O/c1-11-2-4-12(5-3-11)18-14-6-7-23-20(14)24-10-15(18)13-8-16(21)19(25)17(22)9-13/h2-10,25H,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem Lett 28: 3197-3201 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.020
BindingDB Entry DOI: 10.7270/Q2F76G78 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50467096
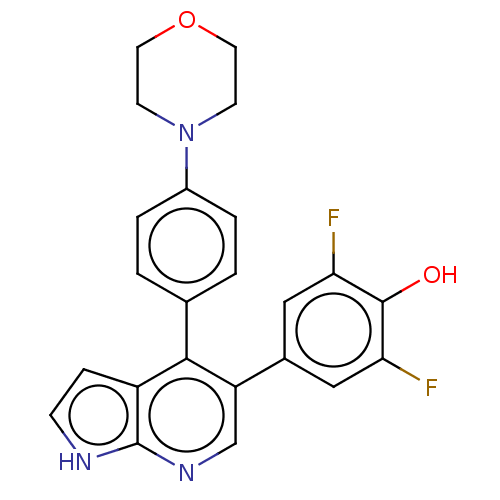
(CHEMBL4278817)Show SMILES Oc1c(F)cc(cc1F)-c1cnc2[nH]ccc2c1-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C23H19F2N3O2/c24-19-11-15(12-20(25)22(19)29)18-13-27-23-17(5-6-26-23)21(18)14-1-3-16(4-2-14)28-7-9-30-10-8-28/h1-6,11-13,29H,7-10H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem Lett 28: 3197-3201 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.020
BindingDB Entry DOI: 10.7270/Q2F76G78 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500936

(CHEMBL3798762)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(n1)-c1nc2cccc(N3CCNCC3)c2n1C Show InChI InChI=1S/C25H28N8O/c1-31(2)25(34)17-7-4-6-16(14-17)19-15-28-23(26)21(29-19)24-30-18-8-5-9-20(22(18)32(24)3)33-12-10-27-11-13-33/h4-9,14-15,27H,10-13H2,1-3H3,(H2,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500933

(CHEMBL3797828)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(c1)-c1nc2cccc(N3CCNCC3)c2[nH]1 Show InChI InChI=1S/C25H27N7O/c1-31(2)25(33)17-6-3-5-16(13-17)18-14-19(23(26)28-15-18)24-29-20-7-4-8-21(22(20)30-24)32-11-9-27-10-12-32/h3-8,13-15,27H,9-12H2,1-2H3,(H2,26,28)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218257
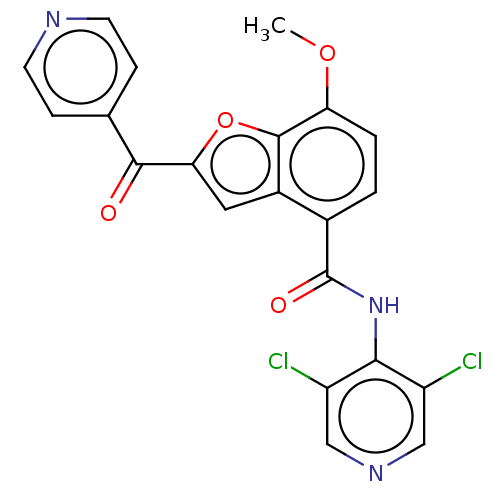
(CHEMBL69874)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(=O)c1ccncc1 Show InChI InChI=1S/C21H13Cl2N3O4/c1-29-16-3-2-12(21(28)26-18-14(22)9-25-10-15(18)23)13-8-17(30-20(13)16)19(27)11-4-6-24-7-5-11/h2-10H,1H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500934
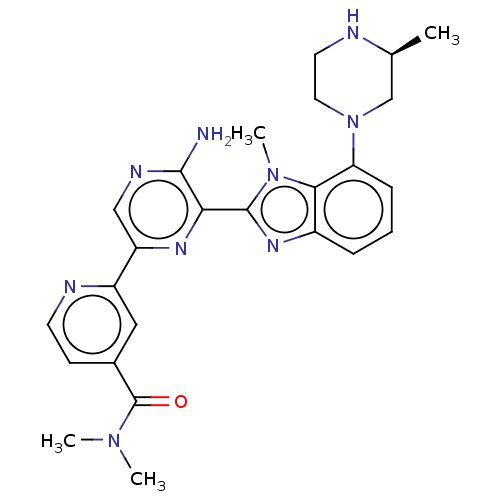
(CHEMBL3800585)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cc(ccn3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C25H29N9O/c1-15-14-34(11-10-27-15)20-7-5-6-17-22(20)33(4)24(31-17)21-23(26)29-13-19(30-21)18-12-16(8-9-28-18)25(35)32(2)3/h5-9,12-13,15,27H,10-11,14H2,1-4H3,(H2,26,29)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50476508

(CHEMBL234487)Show SMILES [#6]-[#8]-c1ccc(cc1-[#7]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#8]-[#6]-[#6]-2)c2ccccc12)[Si;v4]([#6])([#6])[#6] Show InChI InChI=1S/C27H35N3O4Si/c1-32-26-11-9-20(35(2,3)4)19-24(26)29-27(31)28-23-10-12-25(22-8-6-5-7-21(22)23)34-18-15-30-13-16-33-17-14-30/h5-12,19H,13-18H2,1-4H3,(H2,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Paradigm Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MAPK p38 |
Bioorg Med Chem Lett 17: 354-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.044
BindingDB Entry DOI: 10.7270/Q2KD21NQ |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500934

(CHEMBL3800585)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cc(ccn3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C25H29N9O/c1-15-14-34(11-10-27-15)20-7-5-6-17-22(20)33(4)24(31-17)21-23(26)29-13-19(30-21)18-12-16(8-9-28-18)25(35)32(2)3/h5-9,12-13,15,27H,10-11,14H2,1-4H3,(H2,26,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50219014
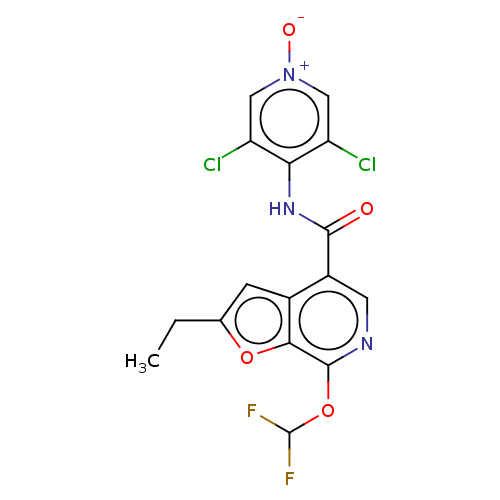
(CHEMBL149559)Show SMILES CCc1cc2c(cnc(OC(F)F)c2o1)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C16H11Cl2F2N3O4/c1-2-7-3-8-9(4-21-15(13(8)26-7)27-16(19)20)14(24)22-12-10(17)5-23(25)6-11(12)18/h3-6,16H,2H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R& D
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 4 from U937 cells |
Bioorg Med Chem Lett 12: 509-12 (2002)
BindingDB Entry DOI: 10.7270/Q2T43W9C |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50500933

(CHEMBL3797828)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(c1)-c1nc2cccc(N3CCNCC3)c2[nH]1 Show InChI InChI=1S/C25H27N7O/c1-31(2)25(33)17-6-3-5-16(13-17)18-14-19(23(26)28-15-18)24-29-20-7-4-8-21(22(20)30-24)32-11-9-27-10-12-32/h3-8,13-15,27H,9-12H2,1-2H3,(H2,26,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111394
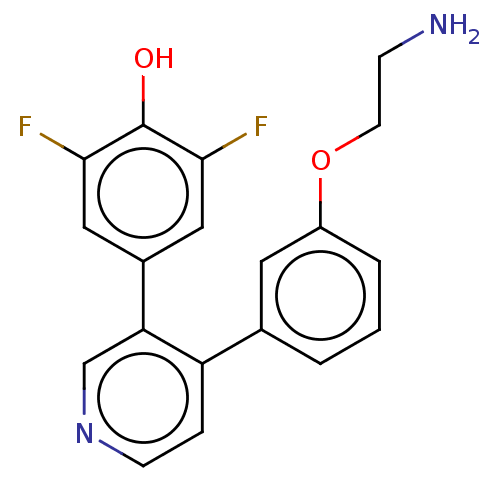
(CHEMBL3604784)Show InChI InChI=1S/C19H16F2N2O2/c20-17-9-13(10-18(21)19(17)24)16-11-23-6-4-15(16)12-2-1-3-14(8-12)25-7-5-22/h1-4,6,8-11,24H,5,7,22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111391

(CHEMBL3604787)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1ccncc1-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C22H21F2N3O/c1-26-7-9-27(10-8-26)17-4-2-3-15(11-17)18-5-6-25-14-19(18)16-12-20(23)22(28)21(24)13-16/h2-6,11-14,28H,7-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111384
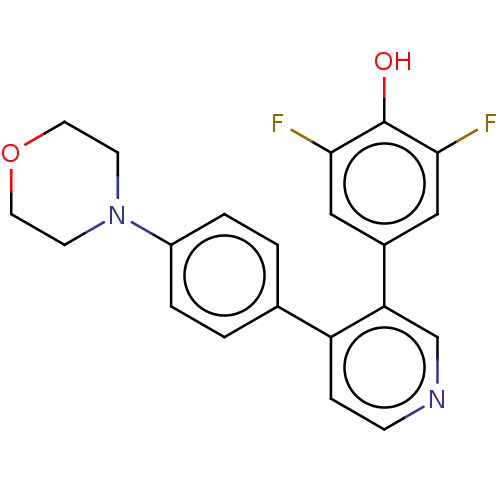
(CHEMBL3604794)Show SMILES Oc1c(F)cc(cc1F)-c1cnccc1-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C21H18F2N2O2/c22-19-11-15(12-20(23)21(19)26)18-13-24-6-5-17(18)14-1-3-16(4-2-14)25-7-9-27-10-8-25/h1-6,11-13,26H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111373
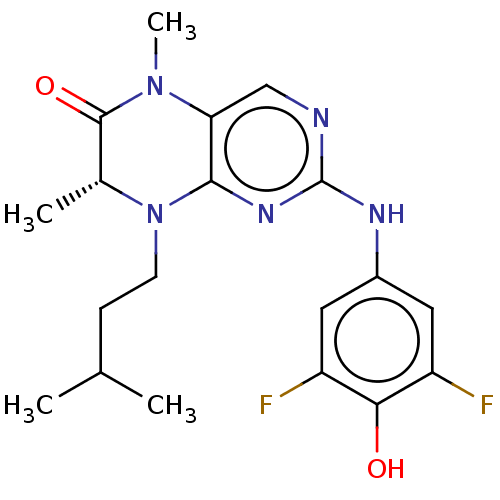
(CHEMBL3604889)Show SMILES CC(C)CCN1[C@H](C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 |r| Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50021658
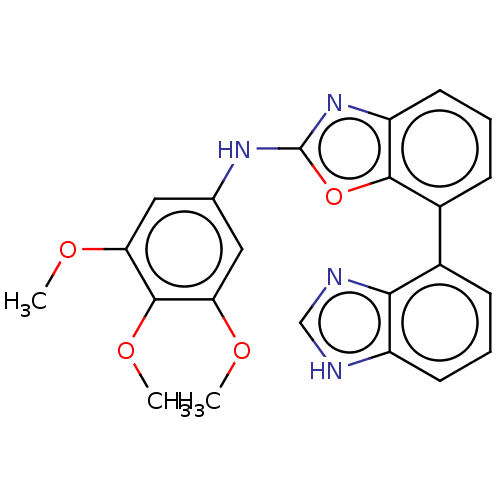
(CHEMBL3298194)Show SMILES COc1cc(Nc2nc3cccc(-c4cccc5[nH]cnc45)c3o2)cc(OC)c1OC Show InChI InChI=1S/C23H20N4O4/c1-28-18-10-13(11-19(29-2)22(18)30-3)26-23-27-17-9-5-7-15(21(17)31-23)14-6-4-8-16-20(14)25-12-24-16/h4-12H,1-3H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length RSK2 (unknown origin) using biotin-AGAGRSRHSSYPAGT-OH as substrate after 150 mins |
Bioorg Med Chem Lett 24: 1592-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.058
BindingDB Entry DOI: 10.7270/Q2V126DB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111384

(CHEMBL3604794)Show SMILES Oc1c(F)cc(cc1F)-c1cnccc1-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C21H18F2N2O2/c22-19-11-15(12-20(23)21(19)26)18-13-24-6-5-17(18)14-1-3-16(4-2-14)25-7-9-27-10-8-25/h1-6,11-13,26H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem Lett 28: 3197-3201 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.020
BindingDB Entry DOI: 10.7270/Q2F76G78 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111385
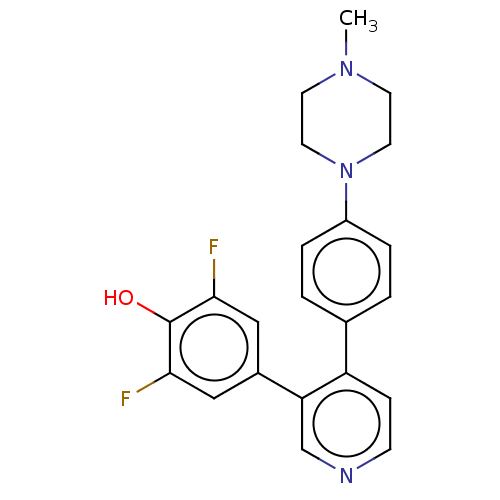
(CHEMBL3604793)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1ccncc1-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C22H21F2N3O/c1-26-8-10-27(11-9-26)17-4-2-15(3-5-17)18-6-7-25-14-19(18)16-12-20(23)22(28)21(24)13-16/h2-7,12-14,28H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem Lett 28: 3197-3201 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.020
BindingDB Entry DOI: 10.7270/Q2F76G78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111385

(CHEMBL3604793)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1ccncc1-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C22H21F2N3O/c1-26-8-10-27(11-9-26)17-4-2-15(3-5-17)18-6-7-25-14-19(18)16-12-20(23)22(28)21(24)13-16/h2-7,12-14,28H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111393
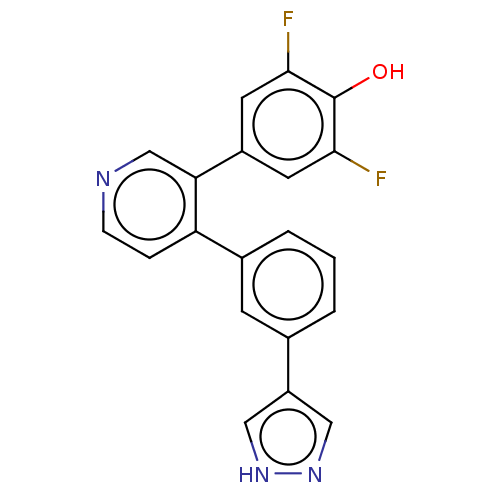
(CHEMBL3604785)Show SMILES Oc1c(F)cc(cc1F)-c1cnccc1-c1cccc(c1)-c1cn[nH]c1 Show InChI InChI=1S/C20H13F2N3O/c21-18-7-14(8-19(22)20(18)26)17-11-23-5-4-16(17)13-3-1-2-12(6-13)15-9-24-25-10-15/h1-11,26H,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111386
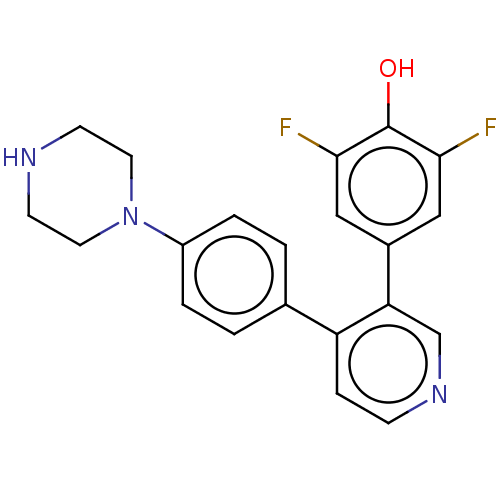
(CHEMBL3604792)Show SMILES Oc1c(F)cc(cc1F)-c1cnccc1-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H19F2N3O/c22-19-11-15(12-20(23)21(19)27)18-13-25-6-5-17(18)14-1-3-16(4-2-14)26-9-7-24-8-10-26/h1-6,11-13,24,27H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111368

(CHEMBL3604775)Show InChI InChI=1S/C15H10F2N2O2/c16-12-5-9(6-13(17)15(12)21)14-11(7-18-19-14)8-1-3-10(20)4-2-8/h1-7,20-21H,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111378
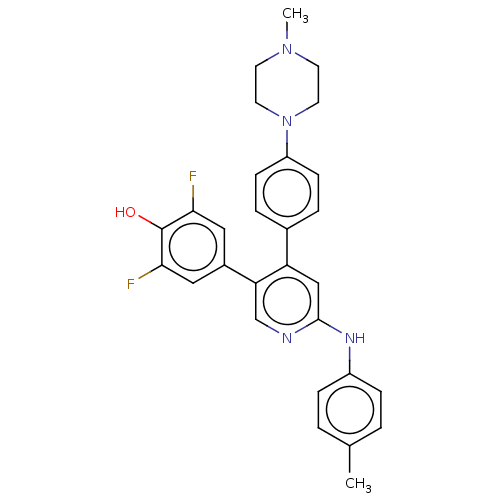
(CHEMBL3604884)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cc(Nc2ccc(C)cc2)ncc1-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C29H28F2N4O/c1-19-3-7-22(8-4-19)33-28-17-24(25(18-32-28)21-15-26(30)29(36)27(31)16-21)20-5-9-23(10-6-20)35-13-11-34(2)12-14-35/h3-10,15-18,36H,11-14H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111381
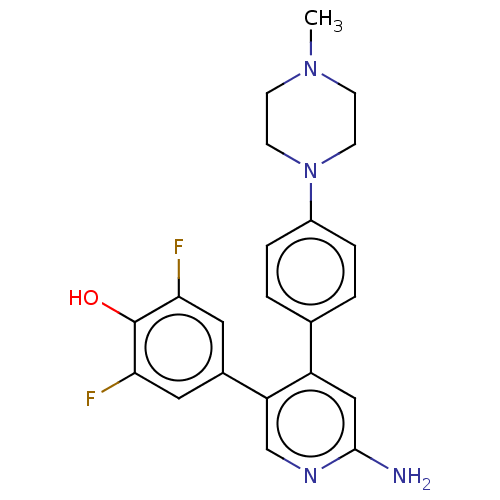
(CHEMBL3604881)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cc(N)ncc1-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C22H22F2N4O/c1-27-6-8-28(9-7-27)16-4-2-14(3-5-16)17-12-21(25)26-13-18(17)15-10-19(23)22(29)20(24)11-15/h2-5,10-13,29H,6-9H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218258
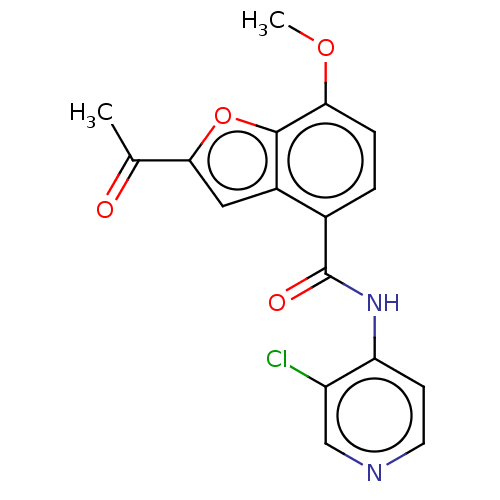
(CHEMBL70146)Show InChI InChI=1S/C17H13ClN2O4/c1-9(21)15-7-11-10(3-4-14(23-2)16(11)24-15)17(22)20-13-5-6-19-8-12(13)18/h3-8H,1-2H3,(H,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50218245
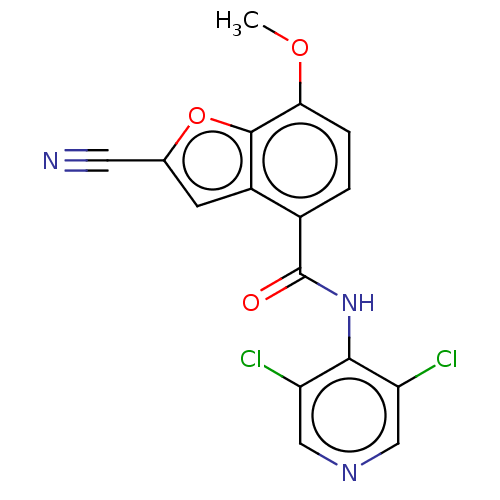
(CHEMBL65799)Show InChI InChI=1S/C16H9Cl2N3O3/c1-23-13-3-2-9(10-4-8(5-19)24-15(10)13)16(22)21-14-11(17)6-20-7-12(14)18/h2-4,6-7H,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rolipram binding to rat brain tissue at 20 uM |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218245

(CHEMBL65799)Show InChI InChI=1S/C16H9Cl2N3O3/c1-23-13-3-2-9(10-4-8(5-19)24-15(10)13)16(22)21-14-11(17)6-20-7-12(14)18/h2-4,6-7H,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111389
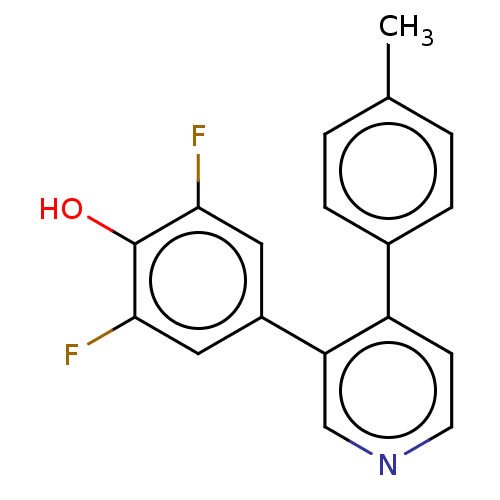
(CHEMBL3604789)Show InChI InChI=1S/C18H13F2NO/c1-11-2-4-12(5-3-11)14-6-7-21-10-15(14)13-8-16(19)18(22)17(20)9-13/h2-10,22H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50476509

(CHEMBL234486)Show SMILES [#6]-c1ccc(cc1)-n1nc(cc1-[#7]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#7]-2-[#6]-[#6]-[#8]-[#6]-[#6]-2)c2ccccc12)[Si;v4]([#6])([#6])[#6] Show InChI InChI=1S/C30H37N5O3Si/c1-22-9-11-23(12-10-22)35-28(21-29(33-35)39(2,3)4)32-30(36)31-26-13-14-27(25-8-6-5-7-24(25)26)38-20-17-34-15-18-37-19-16-34/h5-14,21H,15-20H2,1-4H3,(H2,31,32,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Paradigm Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MAPK p38 |
Bioorg Med Chem Lett 17: 354-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.044
BindingDB Entry DOI: 10.7270/Q2KD21NQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50114418
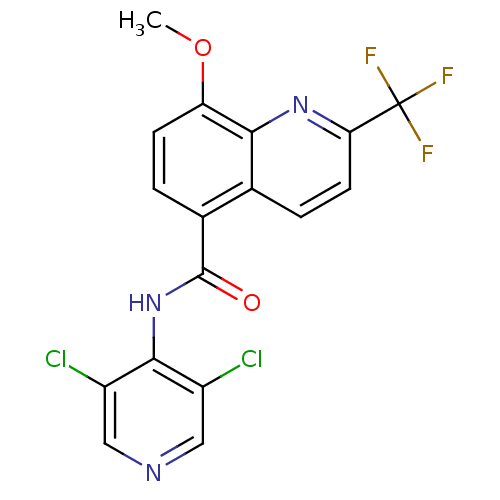
(8-Methoxy-2-trifluoromethyl-quinoline-5-carboxylic...)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H10Cl2F3N3O2/c1-27-12-4-2-9(8-3-5-13(17(20,21)22)24-14(8)12)16(26)25-15-10(18)6-23-7-11(15)19/h2-7H,1H3,(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by ChEMBL
| Assay Description
Inhibition of rolipram binding to PDE4 of rat brain |
Bioorg Med Chem Lett 12: 1621-3 (2002)
BindingDB Entry DOI: 10.7270/Q24J0HB2 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111388
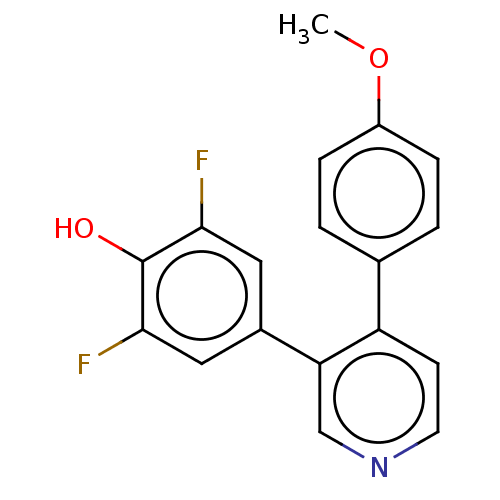
(CHEMBL3604790)Show InChI InChI=1S/C18H13F2NO2/c1-23-13-4-2-11(3-5-13)14-6-7-21-10-15(14)12-8-16(19)18(22)17(20)9-12/h2-10,22H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50218257

(CHEMBL69874)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(=O)c1ccncc1 Show InChI InChI=1S/C21H13Cl2N3O4/c1-29-16-3-2-12(21(28)26-18-14(22)9-25-10-15(18)23)13-8-17(30-20(13)16)19(27)11-4-6-24-7-5-11/h2-10H,1H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rolipram binding to rat brain tissue at 20 uM |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218246
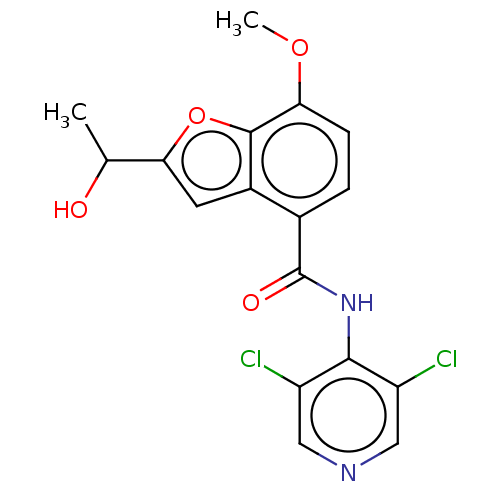
(CHEMBL68323)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(C)O Show InChI InChI=1S/C17H14Cl2N2O4/c1-8(22)14-5-10-9(3-4-13(24-2)16(10)25-14)17(23)21-15-11(18)6-20-7-12(15)19/h3-8,22H,1-2H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Paradigm Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MAPK p38 |
Bioorg Med Chem Lett 17: 354-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.044
BindingDB Entry DOI: 10.7270/Q2KD21NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50213867
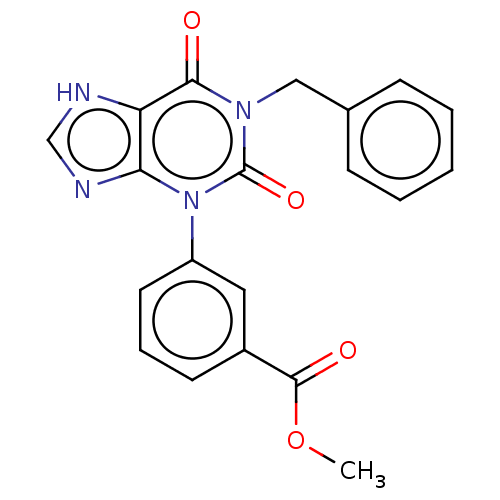
(CHEMBL329303)Show SMILES COC(=O)c1cccc(c1)-n1c2nc[nH]c2c(=O)n(Cc2ccccc2)c1=O Show InChI InChI=1S/C20H16N4O4/c1-28-19(26)14-8-5-9-15(10-14)24-17-16(21-12-22-17)18(25)23(20(24)27)11-13-6-3-2-4-7-13/h2-10,12H,11H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rolipram binding |
Bioorg Med Chem Lett 8: 2925-30 (1998)
BindingDB Entry DOI: 10.7270/Q2Z89FKG |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111377
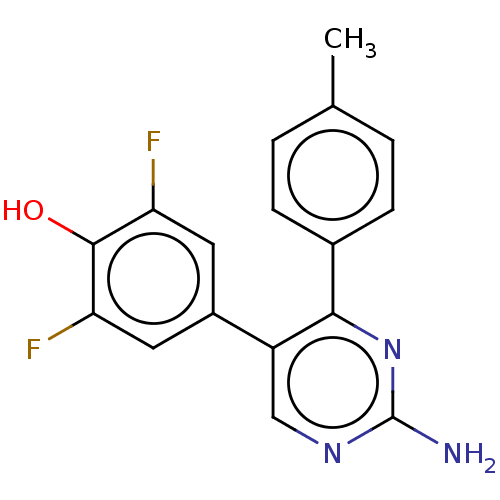
(CHEMBL3604885)Show InChI InChI=1S/C17H13F2N3O/c1-9-2-4-10(5-3-9)15-12(8-21-17(20)22-15)11-6-13(18)16(23)14(19)7-11/h2-8,23H,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50021659
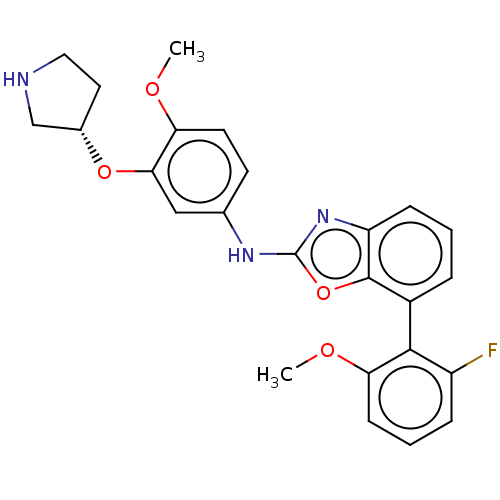
(CHEMBL3297951)Show SMILES COc1ccc(Nc2nc3cccc(-c4c(F)cccc4OC)c3o2)cc1O[C@H]1CCNC1 |r,wU:28.31,(10.88,-23.43,;12.42,-23.43,;13.19,-22.09,;12.42,-20.76,;13.19,-19.42,;14.73,-19.43,;15.5,-18.1,;17.04,-18.1,;17.95,-16.86,;19.41,-17.33,;20.74,-16.56,;22.07,-17.32,;22.08,-18.87,;20.74,-19.65,;20.75,-21.19,;19.42,-21.96,;18.08,-21.18,;19.42,-23.49,;20.75,-24.27,;22.09,-23.49,;22.08,-21.95,;23.41,-21.18,;24.75,-21.94,;19.41,-18.87,;17.94,-19.35,;15.5,-20.76,;14.73,-22.09,;15.51,-23.42,;14.74,-24.76,;15.37,-26.16,;14.23,-27.2,;12.89,-26.43,;13.21,-24.92,)| Show InChI InChI=1S/C25H24FN3O4/c1-30-20-10-9-15(13-22(20)32-16-11-12-27-14-16)28-25-29-19-7-3-5-17(24(19)33-25)23-18(26)6-4-8-21(23)31-2/h3-10,13,16,27H,11-12,14H2,1-2H3,(H,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length RSK2 (unknown origin) using biotin-AGAGRSRHSSYPAGT-OH as substrate after 150 mins |
Bioorg Med Chem Lett 24: 1592-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.058
BindingDB Entry DOI: 10.7270/Q2V126DB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111376
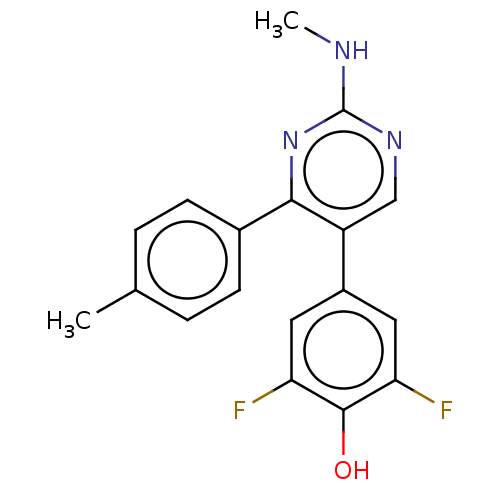
(CHEMBL3604886)Show SMILES CNc1ncc(-c2cc(F)c(O)c(F)c2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C18H15F2N3O/c1-10-3-5-11(6-4-10)16-13(9-22-18(21-2)23-16)12-7-14(19)17(24)15(20)8-12/h3-9,24H,1-2H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111380
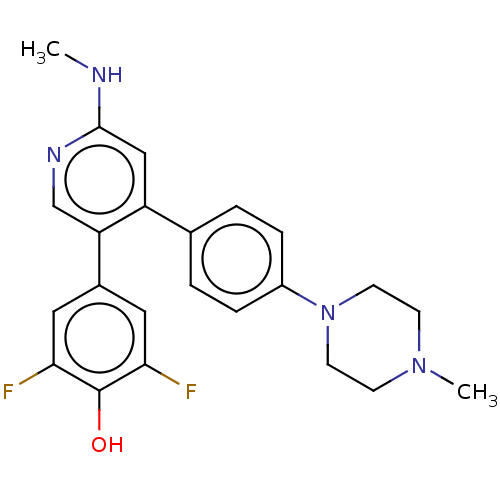
(CHEMBL3604882)Show SMILES CNc1cc(-c2ccc(cc2)N2CCN(C)CC2)c(cn1)-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C23H24F2N4O/c1-26-22-13-18(19(14-27-22)16-11-20(24)23(30)21(25)12-16)15-3-5-17(6-4-15)29-9-7-28(2)8-10-29/h3-6,11-14,30H,7-10H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Histidine-tagged RSK2 using biotin-AGAGRSRHSSYPAGT-OH as substrate preincubated for 30 mins followed by ATP and subst... |
J Med Chem 58: 6766-83 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00450
BindingDB Entry DOI: 10.7270/Q21C1ZN6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50219013
(CHEMBL343205)Show SMILES CCc1cc2c(cnc(OC)c2o1)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C16H13Cl2N3O4/c1-3-8-4-9-10(5-19-16(24-2)14(9)25-8)15(22)20-13-11(17)6-21(23)7-12(13)18/h4-7H,3H2,1-2H3,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R& D
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 4 from U937 cells |
Bioorg Med Chem Lett 12: 509-12 (2002)
BindingDB Entry DOI: 10.7270/Q2T43W9C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data