

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting | ACS Med Chem Lett 7: 283-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00448 BindingDB Entry DOI: 10.7270/Q2D79D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM258470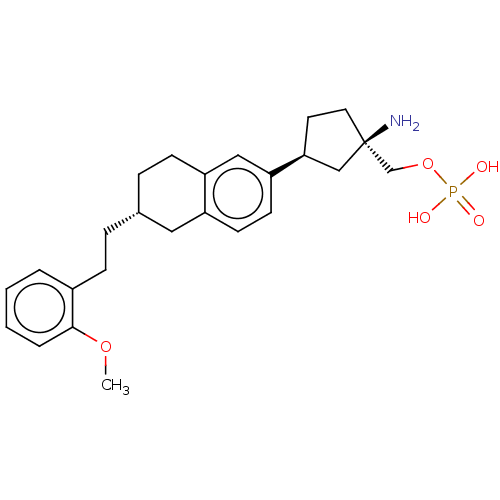 (US9522888, 697) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting | ACS Med Chem Lett 7: 283-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00448 BindingDB Entry DOI: 10.7270/Q2D79D95 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM258466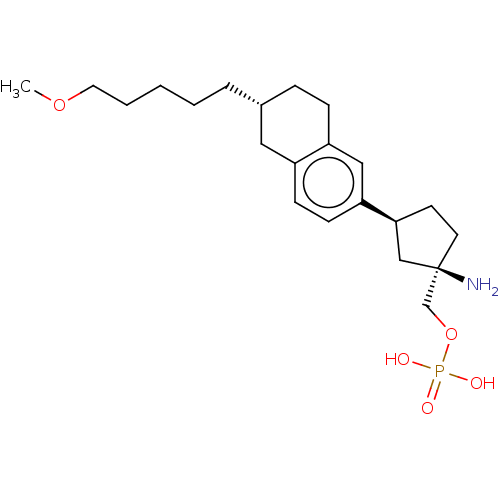 (US9522888, 689) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50562873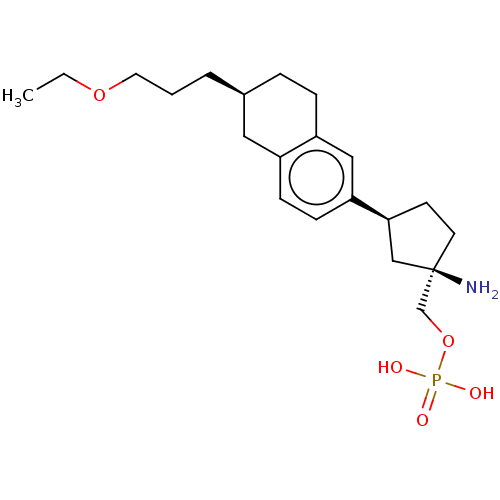 (CHEMBL4786296) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532543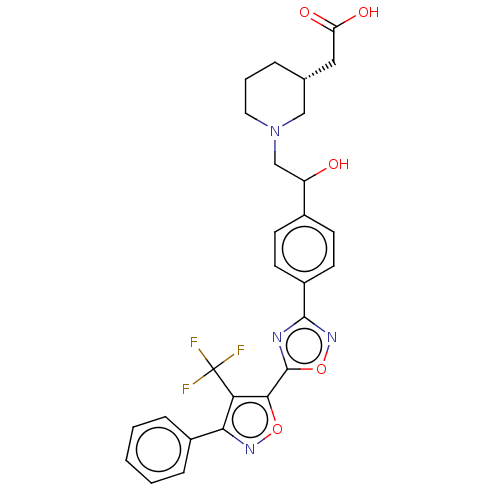 (CHEMBL4474984) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532543 (CHEMBL4474984) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532539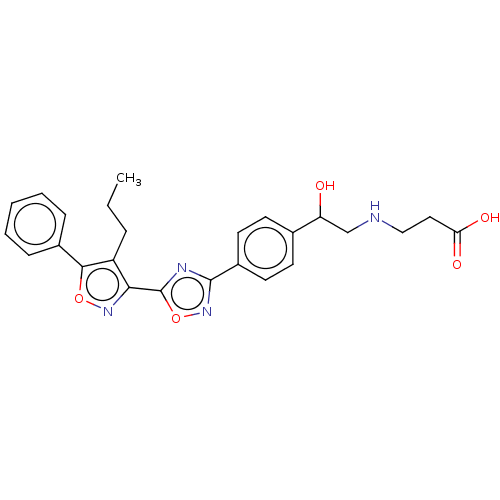 (CHEMBL4569675) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532539 (CHEMBL4569675) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532532 (CHEMBL4457691) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532532 (CHEMBL4457691) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM197654 (US9216972, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human S1P1 | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5CD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532542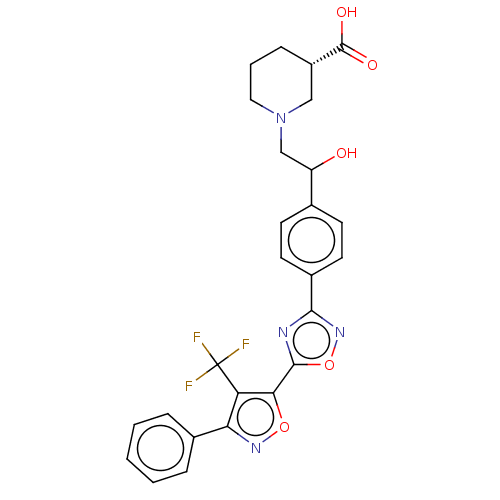 (CHEMBL4461520) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532542 (CHEMBL4461520) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532534 (CHEMBL4469843) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532534 (CHEMBL4469843) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532526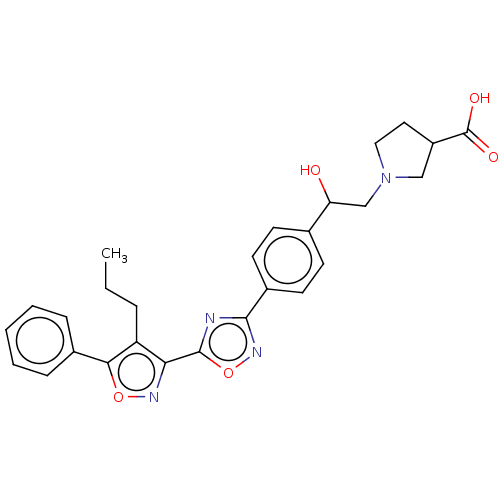 (CHEMBL4440968) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532526 (CHEMBL4440968) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532538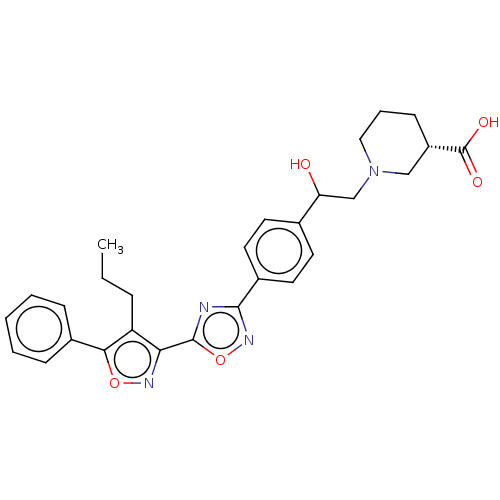 (CHEMBL4587424) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532538 (CHEMBL4587424) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532540 (CHEMBL4475594) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532540 (CHEMBL4475594) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532541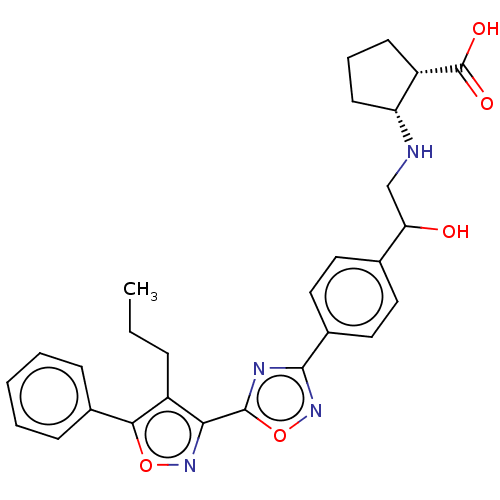 (CHEMBL4452807) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532541 (CHEMBL4452807) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Macaca fascicularis) | BDBM50156282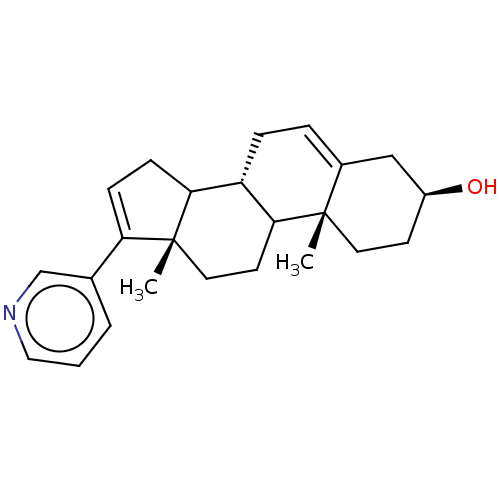 (CHEMBL3780847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156232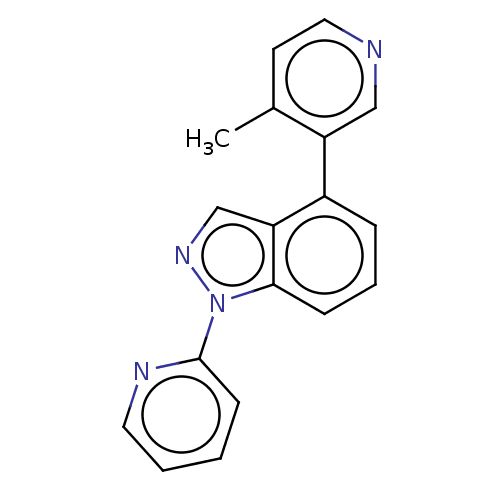 (CHEMBL3782020) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Macaca fascicularis) | BDBM50391846 (CHEMBL2147041 | US9133160, 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156233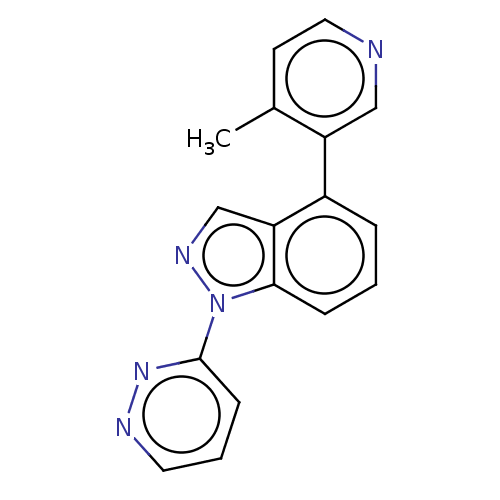 (CHEMBL3780658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Macaca fascicularis) | BDBM50156282 (CHEMBL3780847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156281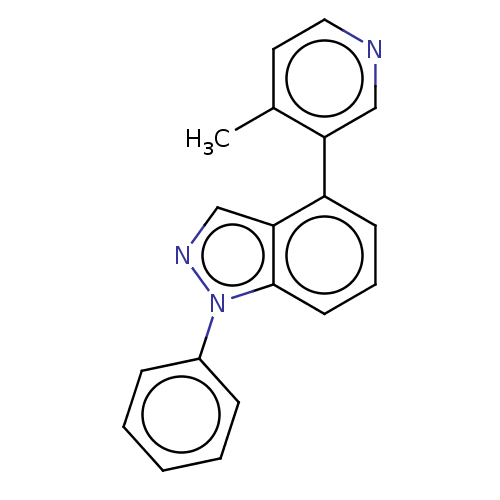 (CHEMBL3780743) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156235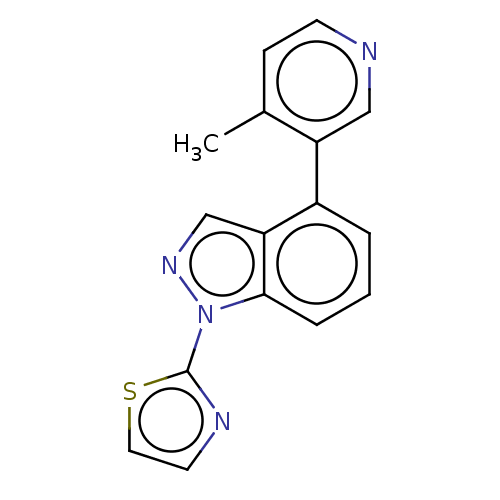 (CHEMBL3780266) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532527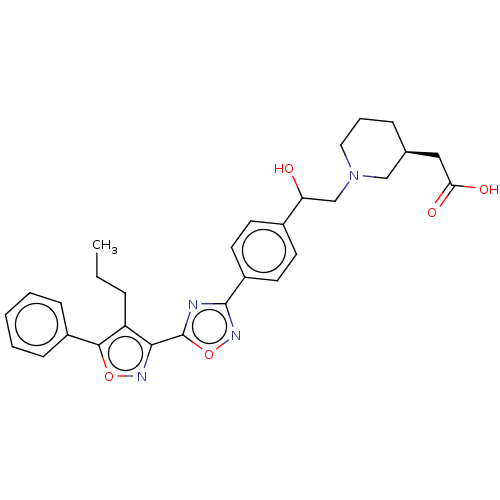 (CHEMBL4464631) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532527 (CHEMBL4464631) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Macaca fascicularis) | BDBM50391846 (CHEMBL2147041 | US9133160, 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156231 (CHEMBL3781112) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156234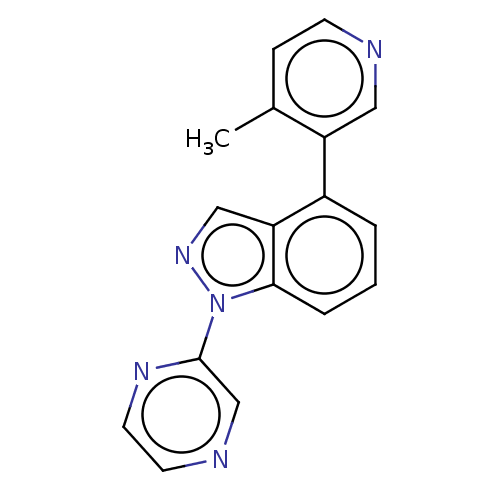 (CHEMBL3780226) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156236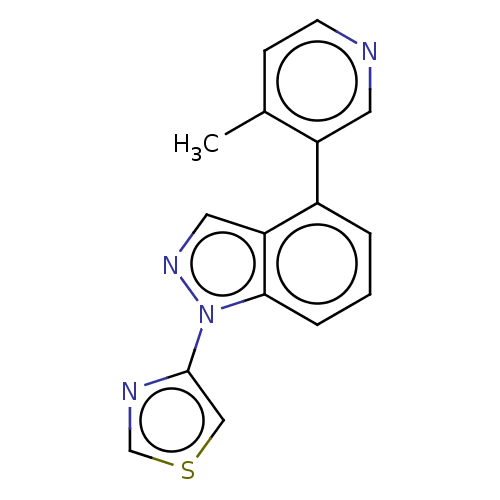 (CHEMBL3780048) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532535 (CHEMBL4545842) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532535 (CHEMBL4545842) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50391839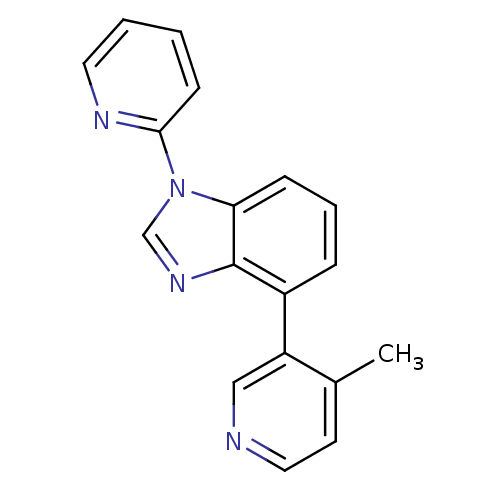 (CHEMBL2147034 | US9133160, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532537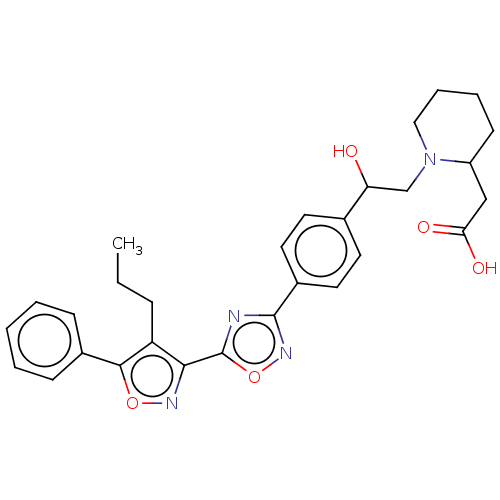 (CHEMBL4553546) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532537 (CHEMBL4553546) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156237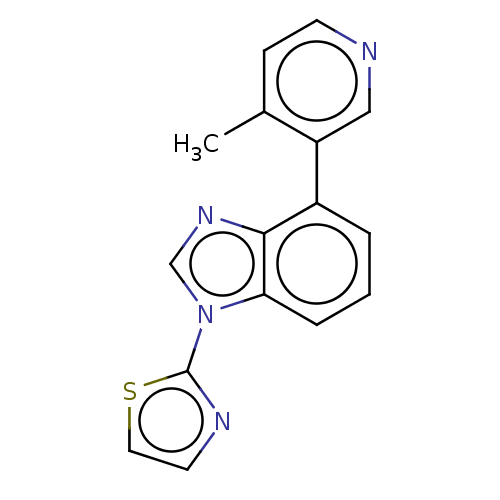 (CHEMBL3781487) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50156238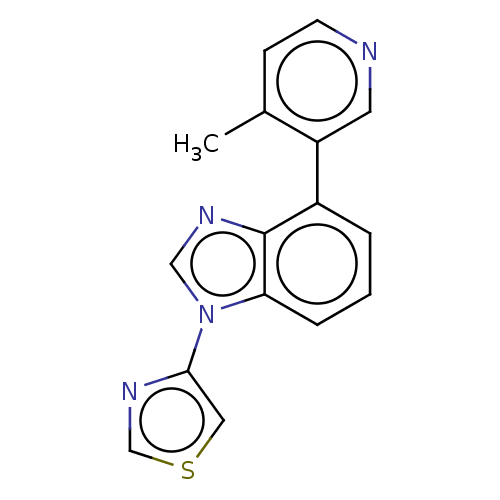 (CHEMBL3781910) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532544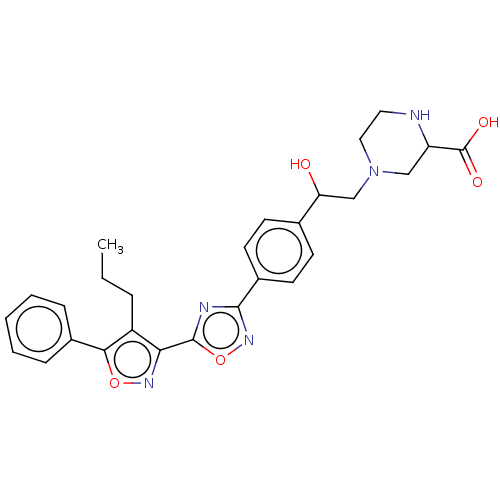 (CHEMBL4553920) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532544 (CHEMBL4553920) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 423 total ) | Next | Last >> |