

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM50022238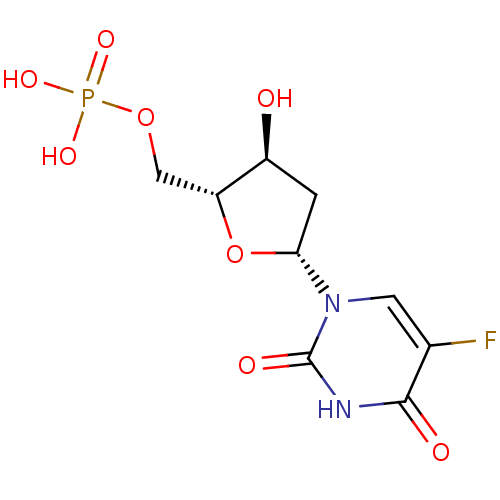 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.00100 | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to amethopterin-resistant Lactobacillus casei thymidylate synthetase in presence of cofactor permitting covalent bond formation | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50022238 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dTMP synthetase with respect to dUMP. | J Med Chem 24: 1161-5 (1982) BindingDB Entry DOI: 10.7270/Q2B56K8B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50022238 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016806 (CHEMBL1236552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010236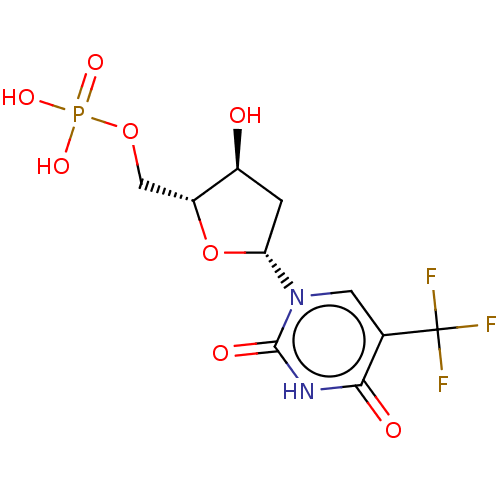 (CHEMBL3144200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010239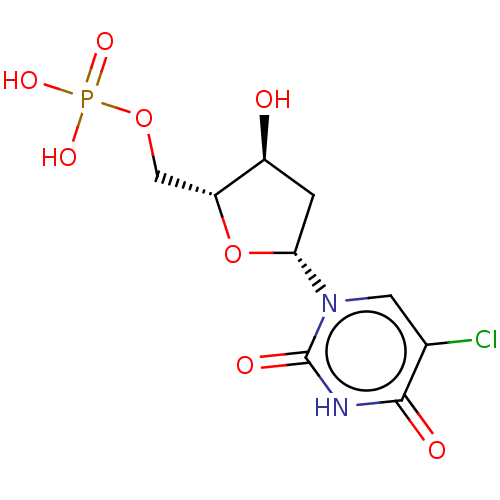 (CHEMBL1236538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50367027 (CHEMBL603555) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dTMP synthetase with respect to dUMP. | J Med Chem 24: 1161-5 (1982) BindingDB Entry DOI: 10.7270/Q2B56K8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010238 (CHEMBL1160593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010242 (CHEMBL1160594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50186267 (2'-deoxy-5'-uridylic acid | CHEMBL211312 | dUMP) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed 5-BrdUMP dehalogenation by absorbance analysis in absence o... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016805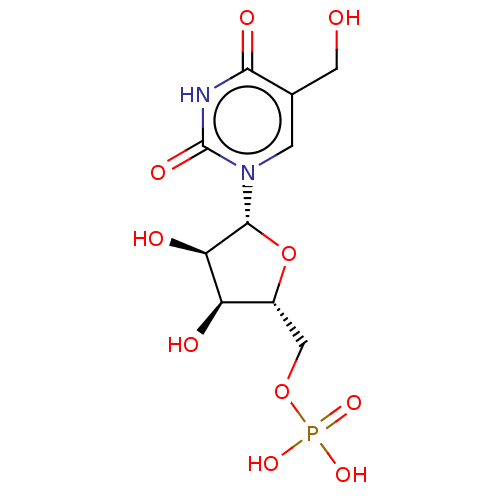 (CHEMBL3274191) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed 5-BrdUMP dehalogenation by absorbance analysis in absence o... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016805 (CHEMBL3274191) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50000965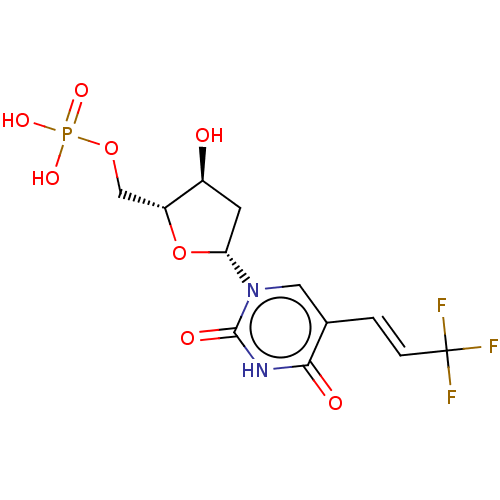 (CHEMBL3228982) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthetase (unknown origin) in presence of 5,10-methylenetetrahydrofolate | J Med Chem 22: 339-40 (1979) BindingDB Entry DOI: 10.7270/Q2183804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50332929 (((2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 8.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed 5-BrdUMP dehalogenation by absorbance analysis in absence o... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010239 (CHEMBL1236538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 9.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed 5-BrdUMP dehalogenation by absorbance analysis in absence o... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50022238 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed 5-BrdUMP dehalogenation by absorbance analysis in absence o... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50332929 (((2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amethopterin-resistant Lactobacillus casei thymidylate synthetase-catalyzed dTMP formation using dUMP as substrate by spectrophotometri... | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50221257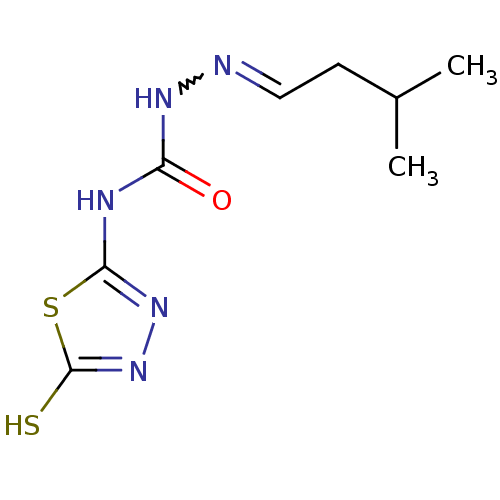 (4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dTMP synthetase with respect to dUMP. | J Med Chem 24: 1161-5 (1982) BindingDB Entry DOI: 10.7270/Q2B56K8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50367025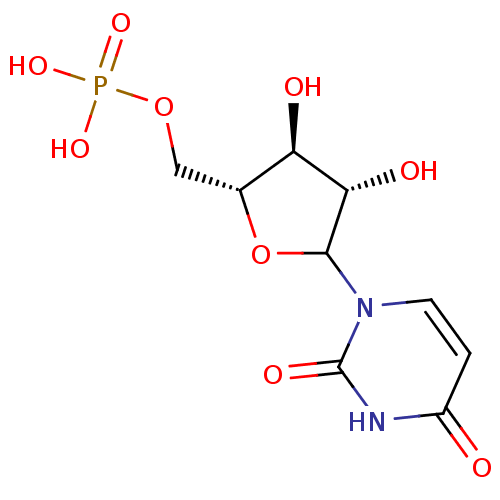 (CHEMBL603336) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dTMP synthetase with respect to dUMP. | J Med Chem 24: 1161-5 (1982) BindingDB Entry DOI: 10.7270/Q2B56K8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50421668 (CHEMBL2311177) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dTMP synthetase with respect to dUMP. | J Med Chem 24: 1161-5 (1982) BindingDB Entry DOI: 10.7270/Q2B56K8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50367024 (CHEMBL606019) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dTMP synthetase with respect to dUMP. | J Med Chem 24: 1161-5 (1982) BindingDB Entry DOI: 10.7270/Q2B56K8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50022238 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to amethopterin-resistant Lactobacillus casei thymidylate synthetase in presence of cofactor not permitting covalent bond formation | J Med Chem 20: 1469-73 (1977) BindingDB Entry DOI: 10.7270/Q2P55Q1K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50000965 (CHEMBL3228982) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to thymidylate synthetase (unknown origin) | J Med Chem 22: 339-40 (1979) BindingDB Entry DOI: 10.7270/Q2183804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||