 Found 24 hits with Last Name = 'wetzel' and Initial = 's'
Found 24 hits with Last Name = 'wetzel' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50323701
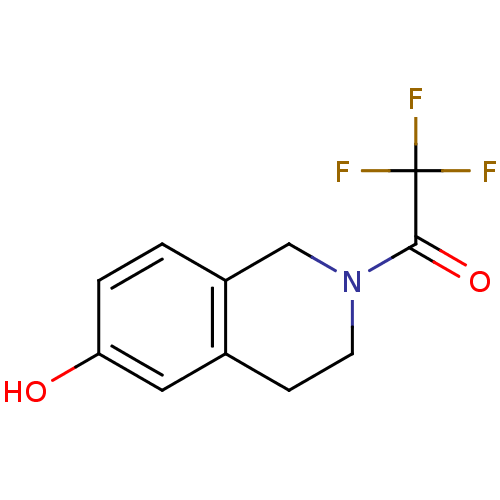
(2-(Trifluoroacetyl)-1,2,3,4-tetrahydro-6-isoquinol...)Show InChI InChI=1S/C11H10F3NO2/c12-11(13,14)10(17)15-4-3-7-5-9(16)2-1-8(7)6-15/h1-2,5,16H,3-4,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human ERalpha ligand binding domain expressed in Escherichia coli BL21 (DE3) |
Nat Chem Biol 5: 585-92 (2009)
Article DOI: 10.1038/nchembio.188
BindingDB Entry DOI: 10.7270/Q2MP53F2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50393328

(CHEMBL2152123)Show InChI InChI=1S/C16H18N2O3/c1-12(18(20)16(17)19)14-7-9-15(10-8-14)21-11-13-5-3-2-4-6-13/h2-10,12,20H,11H2,1H3,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX |
J Med Chem 55: 5989-6001 (2012)
Article DOI: 10.1021/jm300288g
BindingDB Entry DOI: 10.7270/Q2J38TPJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4367
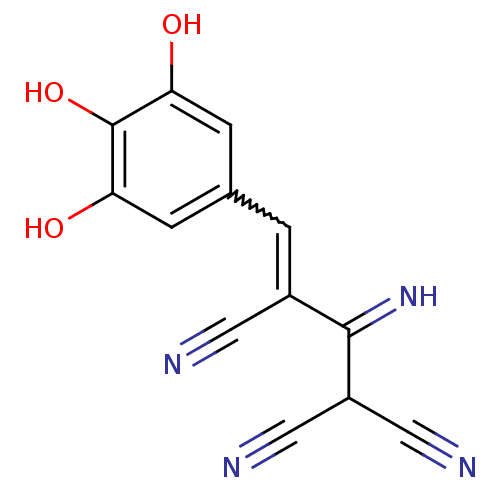
((3Z)-2-amino-3-[(3,4,5-trihydroxyphenyl)methyliden...)Show SMILES Oc1cc(C=C(C#N)C(=N)C(C#N)C#N)cc(O)c1O |w:4.3| Show InChI InChI=1S/C13H8N4O3/c14-4-8(12(17)9(5-15)6-16)1-7-2-10(18)13(20)11(19)3-7/h1-3,9,17-20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem 17: 1079-87 (2009)
Article DOI: 10.1016/j.bmc.2008.02.046
BindingDB Entry DOI: 10.7270/Q2M046C4 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50040420
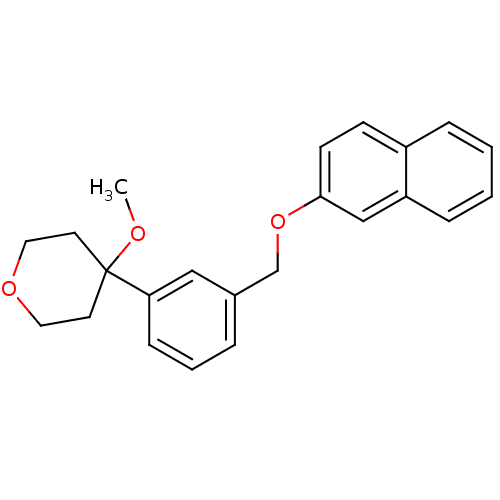
(4-Methoxy-4-[3-(naphthalen-2-yloxymethyl)-phenyl]-...)Show InChI InChI=1S/C23H24O3/c1-24-23(11-13-25-14-12-23)21-8-4-5-18(15-21)17-26-22-10-9-19-6-2-3-7-20(19)16-22/h2-10,15-16H,11-14,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX |
J Med Chem 55: 5989-6001 (2012)
Article DOI: 10.1021/jm300288g
BindingDB Entry DOI: 10.7270/Q2J38TPJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4363
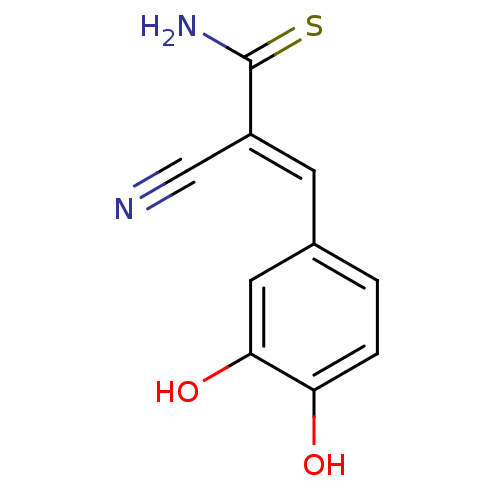
((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enethio...)Show InChI InChI=1S/C10H8N2O2S/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem 17: 1079-87 (2009)
Article DOI: 10.1016/j.bmc.2008.02.046
BindingDB Entry DOI: 10.7270/Q2M046C4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM24941

((2Z)-2-{[(2,5-dibromophenyl)amino](hydroxy)methyli...)Show InChI InChI=1S/C11H8Br2N2O2/c1-6(16)8(5-14)11(17)15-10-4-7(12)2-3-9(10)13/h2-4,8H,1H3,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of BTK |
Bioorg Med Chem 17: 1079-87 (2009)
Article DOI: 10.1016/j.bmc.2008.02.046
BindingDB Entry DOI: 10.7270/Q2M046C4 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50323700
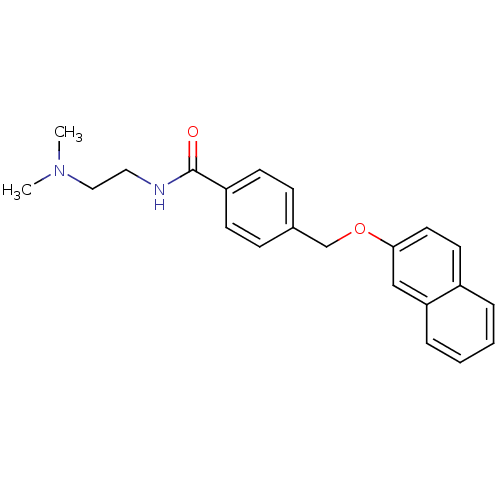
(CHEMBL1213616 | N-(2-(dimethylamino)ethyl)-4-((nap...)Show InChI InChI=1S/C22H24N2O2/c1-24(2)14-13-23-22(25)19-9-7-17(8-10-19)16-26-21-12-11-18-5-3-4-6-20(18)15-21/h3-12,15H,13-14,16H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX |
J Med Chem 55: 5989-6001 (2012)
Article DOI: 10.1021/jm300288g
BindingDB Entry DOI: 10.7270/Q2J38TPJ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50323700

(CHEMBL1213616 | N-(2-(dimethylamino)ethyl)-4-((nap...)Show InChI InChI=1S/C22H24N2O2/c1-24(2)14-13-23-22(25)19-9-7-17(8-10-19)16-26-21-12-11-18-5-3-4-6-20(18)15-21/h3-12,15H,13-14,16H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie
Curated by ChEMBL
| Assay Description
Inhibition of LOX5 in human whole blood assessed as inhibition of conversion of aracidonic acid to leukotrine |
Nat Chem Biol 5: 585-92 (2009)
Article DOI: 10.1038/nchembio.188
BindingDB Entry DOI: 10.7270/Q2MP53F2 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta
(Rattus norvegicus) | BDBM50481906
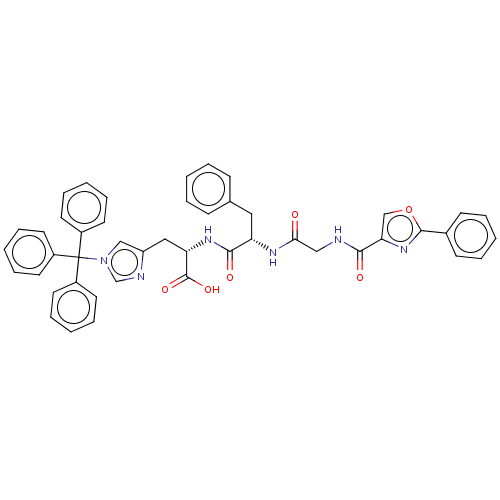
(CHEMBL1075839)Show SMILES OC(=O)[C@H](Cc1cn(cn1)C(c1ccccc1)(c1ccccc1)c1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)c1coc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C46H40N6O6/c53-41(28-47-42(54)40-30-58-44(51-40)33-18-8-2-9-19-33)49-38(26-32-16-6-1-7-17-32)43(55)50-39(45(56)57)27-37-29-52(31-48-37)46(34-20-10-3-11-21-34,35-22-12-4-13-23-35)36-24-14-5-15-25-36/h1-25,29-31,38-39H,26-28H2,(H,47,54)(H,49,53)(H,50,55)(H,56,57)/t38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GGTase-1 by SDS-PAGE end point assay |
J Med Chem 52: 8025-37 (2009)
Article DOI: 10.1021/jm901117d
BindingDB Entry DOI: 10.7270/Q25X2CS3 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM5718
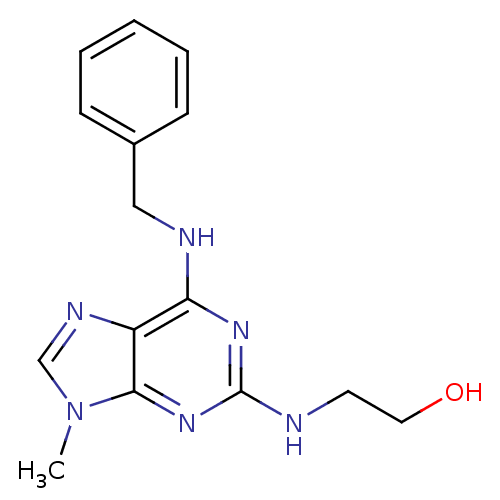
(2,6,9-Trisubstituted purine deriv. 26 | 2-{[6-(ben...)Show InChI InChI=1S/C15H18N6O/c1-21-10-18-12-13(17-9-11-5-3-2-4-6-11)19-15(16-7-8-22)20-14(12)21/h2-6,10,22H,7-9H2,1H3,(H2,16,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E |
Bioorg Med Chem 17: 1079-87 (2009)
Article DOI: 10.1016/j.bmc.2008.02.046
BindingDB Entry DOI: 10.7270/Q2M046C4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM5718

(2,6,9-Trisubstituted purine deriv. 26 | 2-{[6-(ben...)Show InChI InChI=1S/C15H18N6O/c1-21-10-18-12-13(17-9-11-5-3-2-4-6-11)19-15(16-7-8-22)20-14(12)21/h2-6,10,22H,7-9H2,1H3,(H2,16,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin B |
Bioorg Med Chem 17: 1079-87 (2009)
Article DOI: 10.1016/j.bmc.2008.02.046
BindingDB Entry DOI: 10.7270/Q2M046C4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50323699
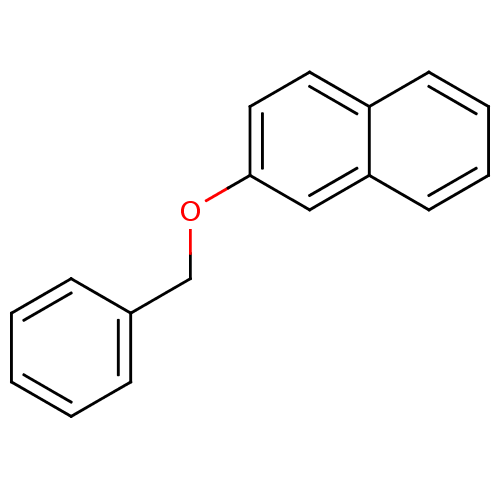
(2-(benzyloxy)naphthalene | 2-Benzyloxy-naphthalene...)Show InChI InChI=1S/C17H14O/c1-2-6-14(7-3-1)13-18-17-11-10-15-8-4-5-9-16(15)12-17/h1-12H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX |
J Med Chem 55: 5989-6001 (2012)
Article DOI: 10.1021/jm300288g
BindingDB Entry DOI: 10.7270/Q2J38TPJ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50323699

(2-(benzyloxy)naphthalene | 2-Benzyloxy-naphthalene...)Show InChI InChI=1S/C17H14O/c1-2-6-14(7-3-1)13-18-17-11-10-15-8-4-5-9-16(15)12-17/h1-12H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie
Curated by ChEMBL
| Assay Description
Inhibition of LOX5 in human whole blood assessed as inhibition of conversion of aracidonic acid to leukotrine |
Nat Chem Biol 5: 585-92 (2009)
Article DOI: 10.1038/nchembio.188
BindingDB Entry DOI: 10.7270/Q2MP53F2 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta
(Rattus norvegicus) | BDBM50481911
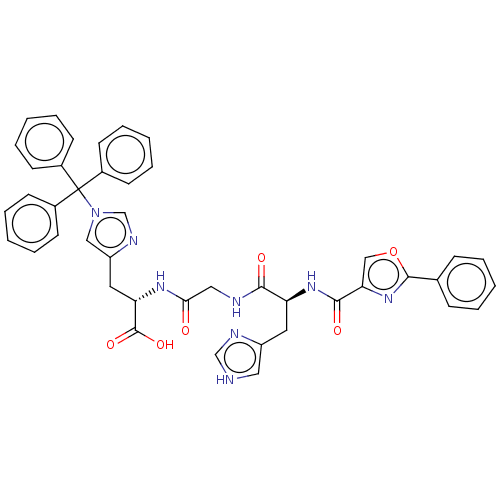
(CHEMBL1075809)Show SMILES OC(=O)[C@H](Cc1cn(cn1)C(c1ccccc1)(c1ccccc1)c1ccccc1)NC(=O)CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)c1coc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C43H38N8O6/c52-38(24-45-39(53)35(21-33-23-44-27-46-33)49-40(54)37-26-57-41(50-37)29-13-5-1-6-14-29)48-36(42(55)56)22-34-25-51(28-47-34)43(30-15-7-2-8-16-30,31-17-9-3-10-18-31)32-19-11-4-12-20-32/h1-20,23,25-28,35-36H,21-22,24H2,(H,44,46)(H,45,53)(H,48,52)(H,49,54)(H,55,56)/t35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GGTase-1 by SDS-PAGE end point assay |
J Med Chem 52: 8025-37 (2009)
Article DOI: 10.1021/jm901117d
BindingDB Entry DOI: 10.7270/Q25X2CS3 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta
(Rattus norvegicus) | BDBM50481907
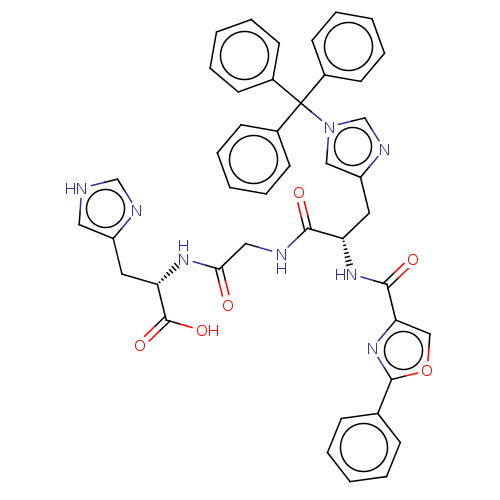
(CHEMBL1075810)Show SMILES OC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CNC(=O)[C@H](Cc1cn(cn1)C(c1ccccc1)(c1ccccc1)c1ccccc1)NC(=O)c1coc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C43H38N8O6/c52-38(48-36(42(55)56)21-33-23-44-27-46-33)24-45-39(53)35(49-40(54)37-26-57-41(50-37)29-13-5-1-6-14-29)22-34-25-51(28-47-34)43(30-15-7-2-8-16-30,31-17-9-3-10-18-31)32-19-11-4-12-20-32/h1-20,23,25-28,35-36H,21-22,24H2,(H,44,46)(H,45,53)(H,48,52)(H,49,54)(H,55,56)/t35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GGTase-1 by SDS-PAGE end point assay |
J Med Chem 52: 8025-37 (2009)
Article DOI: 10.1021/jm901117d
BindingDB Entry DOI: 10.7270/Q25X2CS3 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged Cdc25A expressed in the Escherichia coli BL21(DE3) using p-nitrophenyl phosphate as substrate for 80 mins by spectrophotomet... |
J Med Chem 55: 5989-6001 (2012)
Article DOI: 10.1021/jm300288g
BindingDB Entry DOI: 10.7270/Q2J38TPJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM5718

(2,6,9-Trisubstituted purine deriv. 26 | 2-{[6-(ben...)Show InChI InChI=1S/C15H18N6O/c1-21-10-18-12-13(17-9-11-5-3-2-4-6-11)19-15(16-7-8-22)20-14(12)21/h2-6,10,22H,7-9H2,1H3,(H2,16,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/MAPK |
Bioorg Med Chem 17: 1079-87 (2009)
Article DOI: 10.1016/j.bmc.2008.02.046
BindingDB Entry DOI: 10.7270/Q2M046C4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl transferase type-1 subunit beta
(Rattus norvegicus) | BDBM50481909
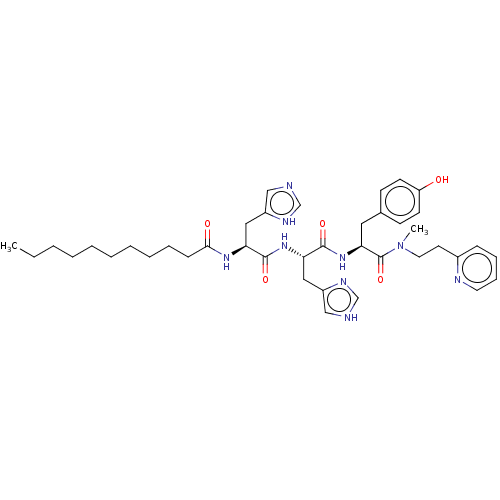
(CHEMBL1075863)Show SMILES CCCCCCCCCCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N(C)CCc1ccccn1 |r| Show InChI InChI=1S/C40H55N9O5/c1-3-4-5-6-7-8-9-10-14-37(51)46-34(23-31-25-41-27-44-31)38(52)47-35(24-32-26-42-28-45-32)39(53)48-36(22-29-15-17-33(50)18-16-29)40(54)49(2)21-19-30-13-11-12-20-43-30/h11-13,15-18,20,25-28,34-36,50H,3-10,14,19,21-24H2,1-2H3,(H,41,44)(H,42,45)(H,46,51)(H,47,52)(H,48,53)/t34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GGTase-1 by SDS-PAGE end point assay |
J Med Chem 52: 8025-37 (2009)
Article DOI: 10.1021/jm901117d
BindingDB Entry DOI: 10.7270/Q25X2CS3 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta
(Rattus norvegicus) | BDBM50481908
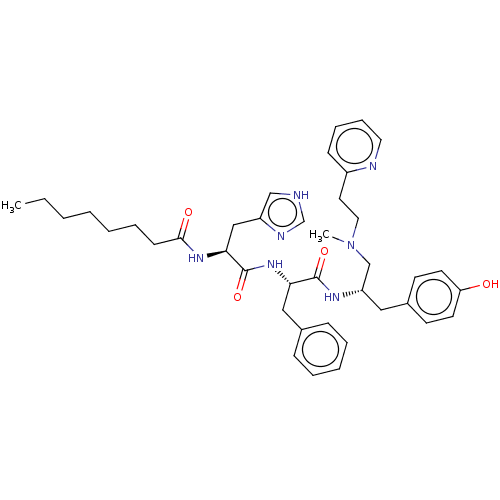
(CHEMBL1075861)Show SMILES CCCCCCCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](CN(C)CCc1ccccn1)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C40H53N7O4/c1-3-4-5-6-10-16-38(49)45-37(26-33-27-41-29-43-33)40(51)46-36(25-30-13-8-7-9-14-30)39(50)44-34(24-31-17-19-35(48)20-18-31)28-47(2)23-21-32-15-11-12-22-42-32/h7-9,11-15,17-20,22,27,29,34,36-37,48H,3-6,10,16,21,23-26,28H2,1-2H3,(H,41,43)(H,44,50)(H,45,49)(H,46,51)/t34-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GGTase-1 by SDS-PAGE end point assay |
J Med Chem 52: 8025-37 (2009)
Article DOI: 10.1021/jm901117d
BindingDB Entry DOI: 10.7270/Q25X2CS3 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta
(Rattus norvegicus) | BDBM50481910
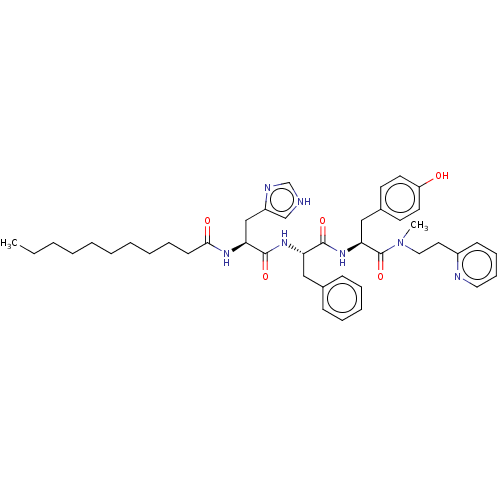
(CHEMBL1075859)Show SMILES CCCCCCCCCCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N(C)CCc1ccccn1 |r| Show InChI InChI=1S/C43H57N7O5/c1-3-4-5-6-7-8-9-13-19-40(52)47-38(29-35-30-44-31-46-35)42(54)48-37(27-32-16-11-10-12-17-32)41(53)49-39(28-33-20-22-36(51)23-21-33)43(55)50(2)26-24-34-18-14-15-25-45-34/h10-12,14-18,20-23,25,30-31,37-39,51H,3-9,13,19,24,26-29H2,1-2H3,(H,44,46)(H,47,52)(H,48,54)(H,49,53)/t37-,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GGTase-1 by SDS-PAGE end point assay |
J Med Chem 52: 8025-37 (2009)
Article DOI: 10.1021/jm901117d
BindingDB Entry DOI: 10.7270/Q25X2CS3 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta
(Rattus norvegicus) | BDBM50481912
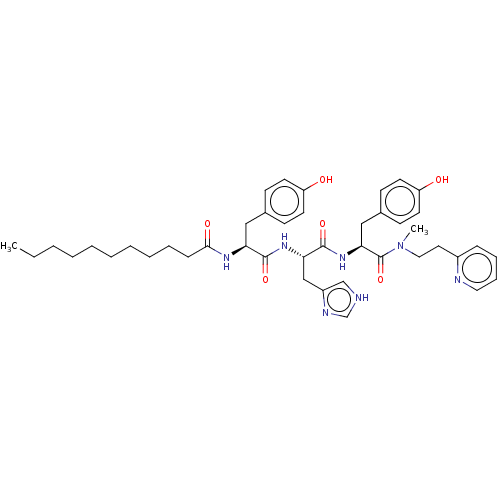
(CHEMBL1075865)Show SMILES CCCCCCCCCCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N(C)CCc1ccccn1 |r| Show InChI InChI=1S/C43H57N7O6/c1-3-4-5-6-7-8-9-10-14-40(53)47-37(26-31-15-19-35(51)20-16-31)41(54)48-38(28-34-29-44-30-46-34)42(55)49-39(27-32-17-21-36(52)22-18-32)43(56)50(2)25-23-33-13-11-12-24-45-33/h11-13,15-22,24,29-30,37-39,51-52H,3-10,14,23,25-28H2,1-2H3,(H,44,46)(H,47,53)(H,48,54)(H,49,55)/t37-,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GGTase-1 by SDS-PAGE end point assay |
J Med Chem 52: 8025-37 (2009)
Article DOI: 10.1021/jm901117d
BindingDB Entry DOI: 10.7270/Q25X2CS3 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM4363

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enethio...)Show InChI InChI=1S/C10H8N2O2S/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of IRK |
Bioorg Med Chem 17: 1079-87 (2009)
Article DOI: 10.1016/j.bmc.2008.02.046
BindingDB Entry DOI: 10.7270/Q2M046C4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50323701

(2-(Trifluoroacetyl)-1,2,3,4-tetrahydro-6-isoquinol...)Show InChI InChI=1S/C11H10F3NO2/c12-11(13,14)10(17)15-4-3-7-5-9(16)2-1-8(7)6-15/h1-2,5,16H,3-4,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie
Curated by ChEMBL
| Assay Description
Binding affinity to human ERbeta ligand binding domain expressed in Escherichia coli BL21(DE3) cells assessed as recruitment of fluorescently labeled... |
Nat Chem Biol 5: 585-92 (2009)
Article DOI: 10.1038/nchembio.188
BindingDB Entry DOI: 10.7270/Q2MP53F2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50323701

(2-(Trifluoroacetyl)-1,2,3,4-tetrahydro-6-isoquinol...)Show InChI InChI=1S/C11H10F3NO2/c12-11(13,14)10(17)15-4-3-7-5-9(16)2-1-8(7)6-15/h1-2,5,16H,3-4,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 275 | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie
Curated by ChEMBL
| Assay Description
Binding affinity to human ERalpha ligand binding domain expressed in Escherichia coli BL21(DE3) cells assessed as recruitment of fluorescently labele... |
Nat Chem Biol 5: 585-92 (2009)
Article DOI: 10.1038/nchembio.188
BindingDB Entry DOI: 10.7270/Q2MP53F2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data