 Found 4708 hits with Last Name = 'white' and Initial = 'c'
Found 4708 hits with Last Name = 'white' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
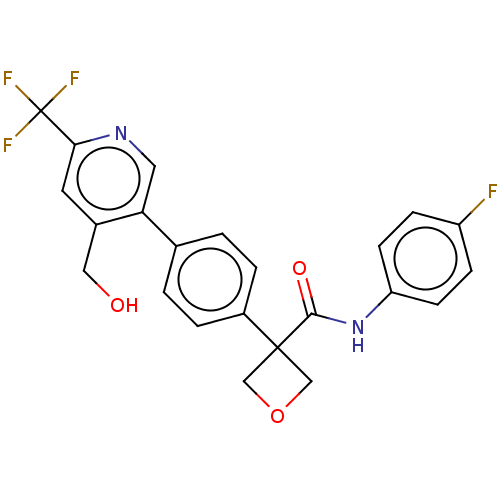
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180524
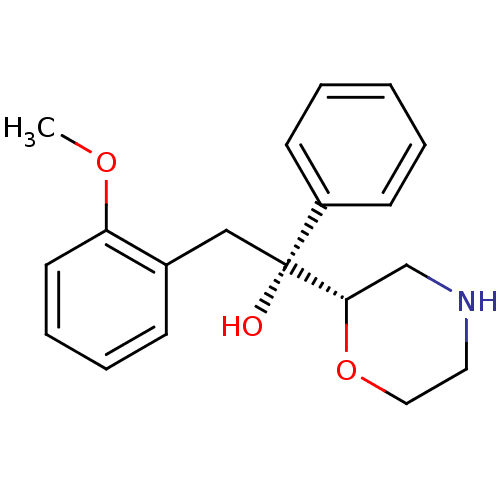
((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...)Show InChI InChI=1S/C19H23NO3/c1-22-17-10-6-5-7-15(17)13-19(21,16-8-3-2-4-9-16)18-14-20-11-12-23-18/h2-10,18,20-21H,11-14H2,1H3/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180521
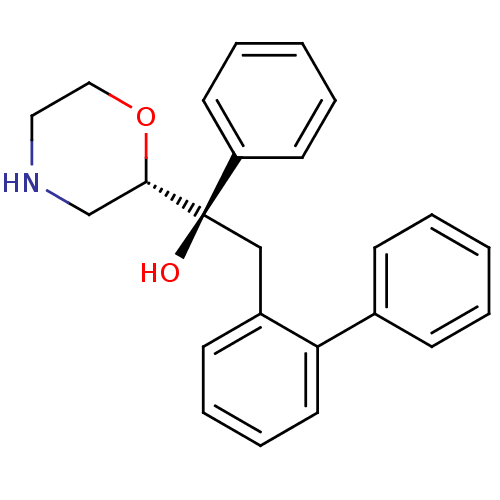
((R)-2-biphenyl-2-yl-1-(S)-morpholin-2-yl-1-phenyl-...)Show SMILES O[C@@](Cc1ccccc1-c1ccccc1)([C@@H]1CNCCO1)c1ccccc1 Show InChI InChI=1S/C24H25NO2/c26-24(21-12-5-2-6-13-21,23-18-25-15-16-27-23)17-20-11-7-8-14-22(20)19-9-3-1-4-10-19/h1-14,23,25-26H,15-18H2/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180531

((R)-2-(2-ethoxyphenyl)-1-((S)-morpholin-2-yl)-1-ph...)Show InChI InChI=1S/C20H25NO3/c1-2-23-18-11-7-6-8-16(18)14-20(22,17-9-4-3-5-10-17)19-15-21-12-13-24-19/h3-11,19,21-22H,2,12-15H2,1H3/t19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180526
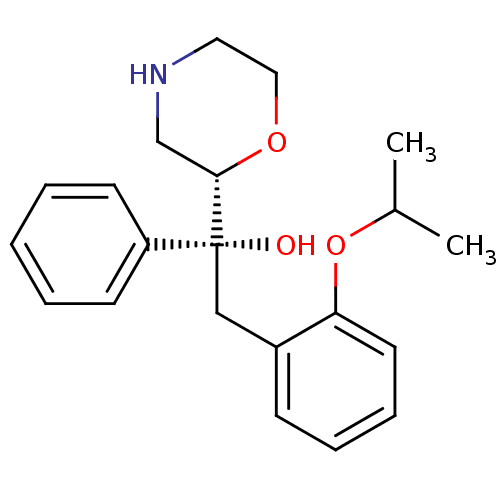
((R)-2-(2-isopropoxyphenyl)-1-((S)-morpholin-2-yl)-...)Show SMILES CC(C)Oc1ccccc1C[C@](O)([C@@H]1CNCCO1)c1ccccc1 Show InChI InChI=1S/C21H27NO3/c1-16(2)25-19-11-7-6-8-17(19)14-21(23,18-9-4-3-5-10-18)20-15-22-12-13-24-20/h3-11,16,20,22-23H,12-15H2,1-2H3/t20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180526

((R)-2-(2-isopropoxyphenyl)-1-((S)-morpholin-2-yl)-...)Show SMILES CC(C)Oc1ccccc1C[C@](O)([C@@H]1CNCCO1)c1ccccc1 Show InChI InChI=1S/C21H27NO3/c1-16(2)25-19-11-7-6-8-17(19)14-21(23,18-9-4-3-5-10-18)20-15-22-12-13-24-20/h3-11,16,20,22-23H,12-15H2,1-2H3/t20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180523
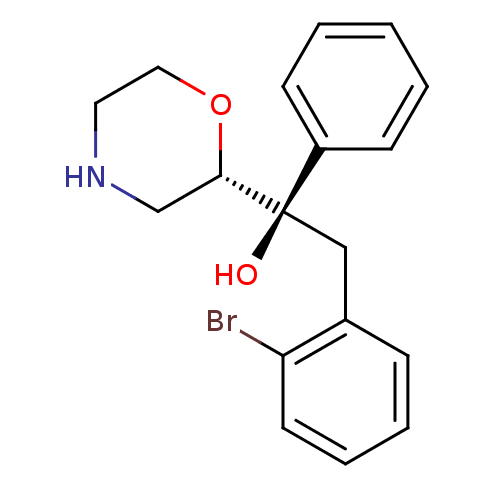
((R)-2-(2-bromophenyl)-1-((S)-morpholin-2-yl)-1-phe...)Show InChI InChI=1S/C18H20BrNO2/c19-16-9-5-4-6-14(16)12-18(21,15-7-2-1-3-8-15)17-13-20-10-11-22-17/h1-9,17,20-21H,10-13H2/t17-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180525

((R)-2-(2-chlorophenyl)-1-((S)-morpholin-2-yl)-1-ph...)Show InChI InChI=1S/C18H20ClNO2/c19-16-9-5-4-6-14(16)12-18(21,15-7-2-1-3-8-15)17-13-20-10-11-22-17/h1-9,17,20-21H,10-13H2/t17-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180522
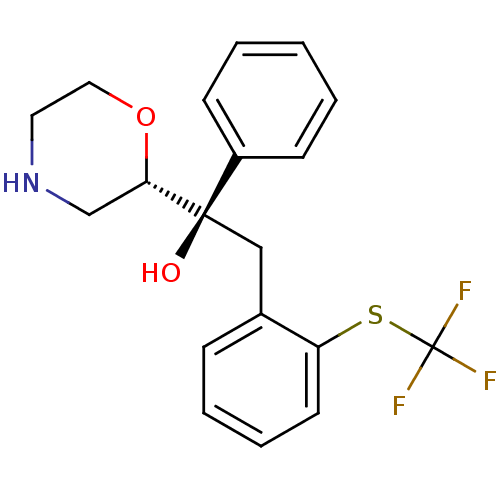
((R)-1-((S)-morpholin-2-yl)-1-phenyl-2-(2-(trifluor...)Show SMILES O[C@@](Cc1ccccc1SC(F)(F)F)([C@@H]1CNCCO1)c1ccccc1 Show InChI InChI=1S/C19H20F3NO2S/c20-19(21,22)26-16-9-5-4-6-14(16)12-18(24,15-7-2-1-3-8-15)17-13-23-10-11-25-17/h1-9,17,23-24H,10-13H2/t17-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 54.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180524

((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...)Show InChI InChI=1S/C19H23NO3/c1-22-17-10-6-5-7-15(17)13-19(21,16-8-3-2-4-9-16)18-14-20-11-12-23-18/h2-10,18,20-21H,11-14H2,1H3/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85002
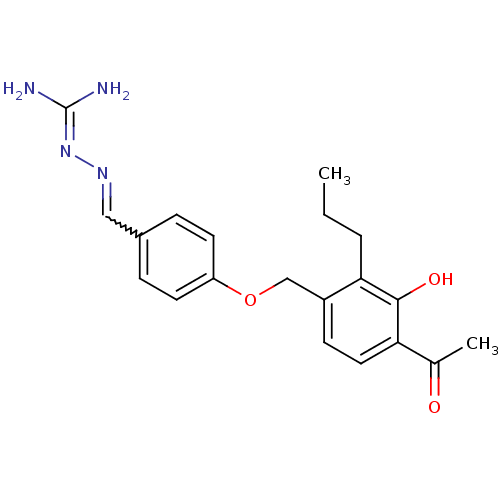
(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85006
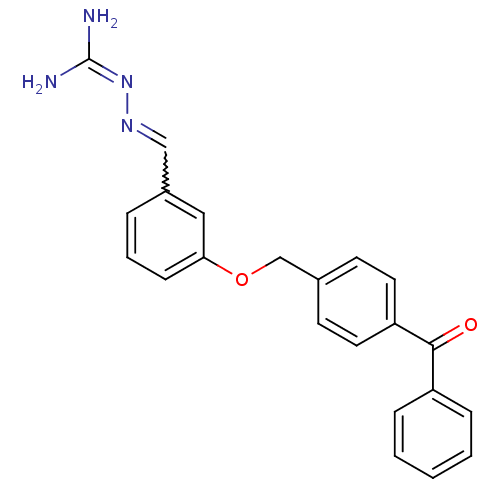
(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85007
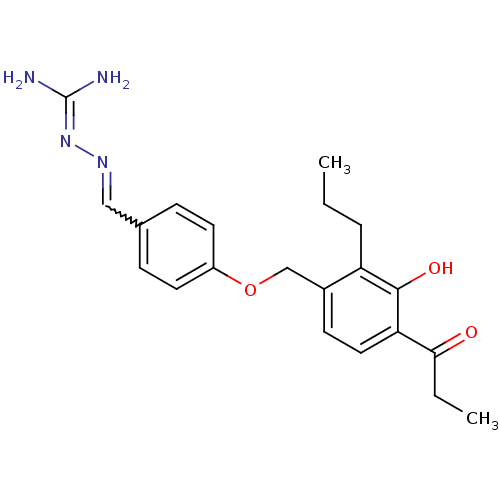
(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85000
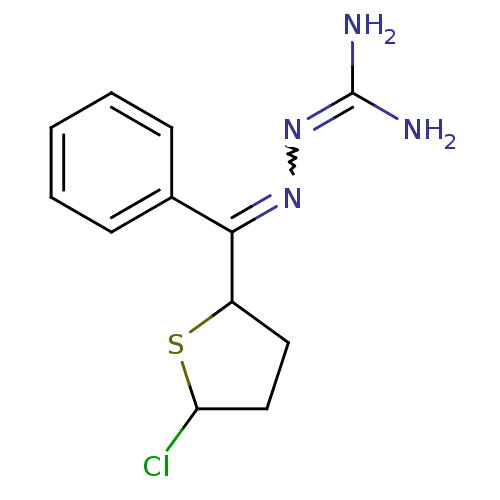
(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180527

((R)-1-((S)-morpholin-2-yl)-1,2-diphenylethanol | C...)Show InChI InChI=1S/C18H21NO2/c20-18(16-9-5-2-6-10-16,17-14-19-11-12-21-17)13-15-7-3-1-4-8-15/h1-10,17,19-20H,11-14H2/t17-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180524

((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...)Show InChI InChI=1S/C19H23NO3/c1-22-17-10-6-5-7-15(17)13-19(21,16-8-3-2-4-9-16)18-14-20-11-12-23-18/h2-10,18,20-21H,11-14H2,1H3/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50180524

((R)-2-(2-methoxyphenyl)-1-((S)-morpholin-2-yl)-1-p...)Show InChI InChI=1S/C19H23NO3/c1-22-17-10-6-5-7-15(17)13-19(21,16-8-3-2-4-9-16)18-14-20-11-12-23-18/h2-10,18,20-21H,11-14H2,1H3/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity to NET |
Bioorg Med Chem Lett 16: 2022-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.061
BindingDB Entry DOI: 10.7270/Q21J9BJ5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85002

(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85006

(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85004
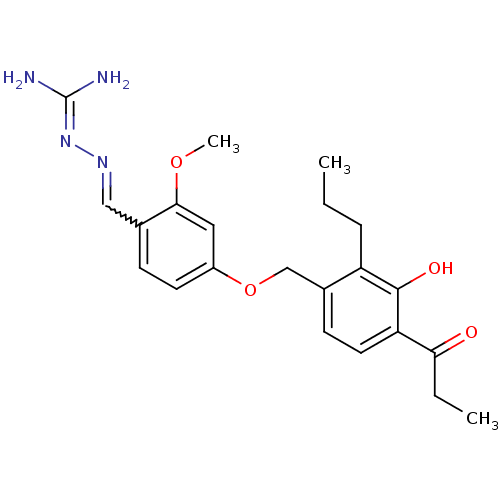
(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85004

(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85007

(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85003
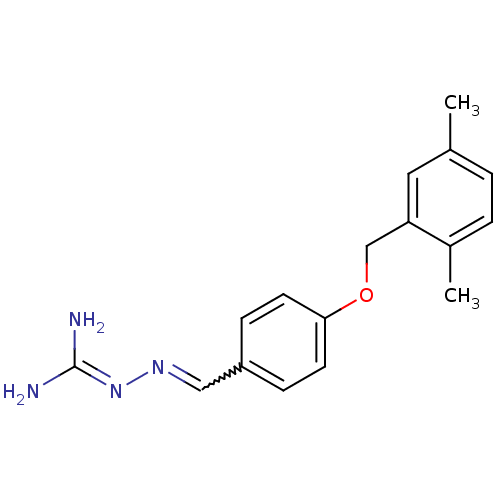
(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85003

(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85004

(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85000

(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85006

(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 477 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Rattus norvegicus) | BDBM50478562

(CHEMBL502491)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(OC)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)CCCCCCCCCCCCC)[C@@]1(OC(C)=O)C2(C)C |r,c:15,t:4| Show InChI InChI=1S/C37H58O8/c1-8-9-10-11-12-13-14-15-16-17-18-19-30(40)44-33-25(3)36(42)28(31-34(5,6)37(31,33)45-26(4)39)21-27(23-38)22-35(43-7)29(36)20-24(2)32(35)41/h20-21,25,28-29,31,33,38,42H,8-19,22-23H2,1-7H3/t25-,28+,29-,31-,33-,35-,36-,37-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled PDBU from PKC in rat forebrain |
J Nat Prod 54: 1440-3
Article DOI: 10.1021/np50077a040
BindingDB Entry DOI: 10.7270/Q2377CGF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85003

(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85001
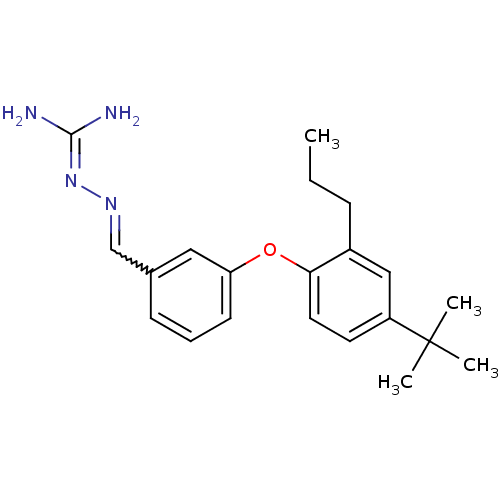
(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 839 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85001

(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85002

(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85007

(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85000

(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85001

(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85005
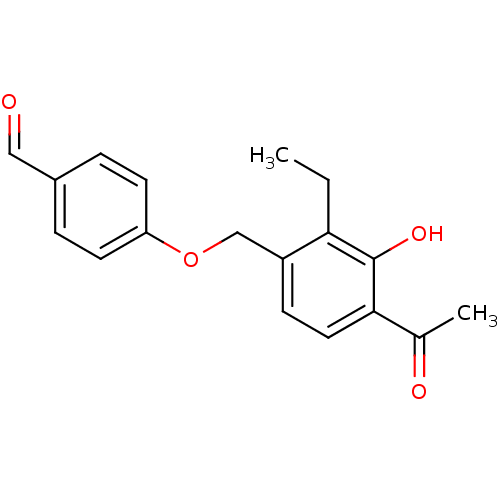
(LY 335102)Show InChI InChI=1S/C18H18O4/c1-3-16-14(6-9-17(12(2)20)18(16)21)11-22-15-7-4-13(10-19)5-8-15/h4-10,21H,3,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85005

(LY 335102)Show InChI InChI=1S/C18H18O4/c1-3-16-14(6-9-17(12(2)20)18(16)21)11-22-15-7-4-13(10-19)5-8-15/h4-10,21H,3,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Superoxide dismutase [Cu-Zn]
(Bos taurus (Cattle)) | BDBM241955

(Aβ (1-40) | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIG...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(C)C)C(C)C)[C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhodes University
| Assay Description
Purified SOD from bovine brain (20 μl) was incubated (37°C, 60 min) with Aβ25-37, Aβ29-33 or Aβ1-40 (20 μl, 5 μM) follo... |
J Enzyme Inhib Med Chem 28: 727-33 (2013)
Article DOI: 10.3109/14756366.2012.680063
BindingDB Entry DOI: 10.7270/Q2JM28KG |
More data for this
Ligand-Target Pair | |
Superoxide dismutase [Cu-Zn]
(Bos taurus (Cattle)) | BDBM241954

(Aβ (29-33) | GAIIG)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CN)[C@@H](C)CC)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C19H35N5O6/c1-6-10(3)15(18(29)21-9-14(26)27)24-19(30)16(11(4)7-2)23-17(28)12(5)22-13(25)8-20/h10-12,15-16H,6-9,20H2,1-5H3,(H,21,29)(H,22,25)(H,23,28)(H,24,30)(H,26,27)/t10-,11-,12-,15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhodes University
| Assay Description
Purified SOD from bovine brain (20 μl) was incubated (37°C, 60 min) with Aβ25-37, Aβ29-33 or Aβ1-40 (20 μl, 5 μM) follo... |
J Enzyme Inhib Med Chem 28: 727-33 (2013)
Article DOI: 10.3109/14756366.2012.680063
BindingDB Entry DOI: 10.7270/Q2JM28KG |
More data for this
Ligand-Target Pair | |
Superoxide dismutase [Cu-Zn]
(Bos taurus (Cattle)) | BDBM241953
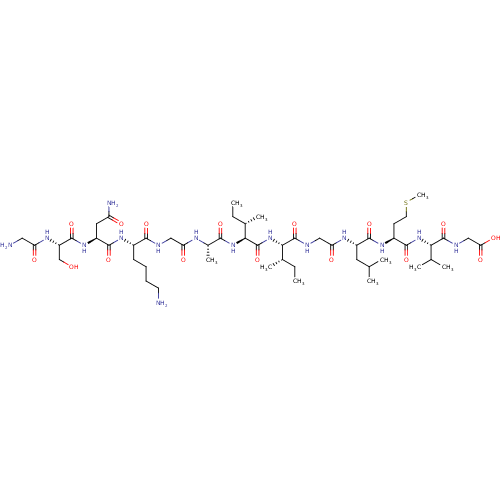
(Aβ (25-37) | GSNKGAIIGLMVG)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)CN)[C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C52H93N15O16S/c1-11-28(7)42(51(82)57-23-39(72)60-33(19-26(3)4)47(78)63-32(16-18-84-10)46(77)65-41(27(5)6)50(81)58-24-40(73)74)67-52(83)43(29(8)12-2)66-44(75)30(9)59-38(71)22-56-45(76)31(15-13-14-17-53)62-48(79)34(20-36(55)69)64-49(80)35(25-68)61-37(70)21-54/h26-35,41-43,68H,11-25,53-54H2,1-10H3,(H2,55,69)(H,56,76)(H,57,82)(H,58,81)(H,59,71)(H,60,72)(H,61,70)(H,62,79)(H,63,78)(H,64,80)(H,65,77)(H,66,75)(H,67,83)(H,73,74)/t28-,29-,30-,31-,32-,33-,34-,35-,41-,42-,43-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhodes University
| Assay Description
Purified SOD from bovine brain (20 μl) was incubated (37°C, 60 min) with Aβ25-37, Aβ29-33 or Aβ1-40 (20 μl, 5 μM) follo... |
J Enzyme Inhib Med Chem 28: 727-33 (2013)
Article DOI: 10.3109/14756366.2012.680063
BindingDB Entry DOI: 10.7270/Q2JM28KG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50567201
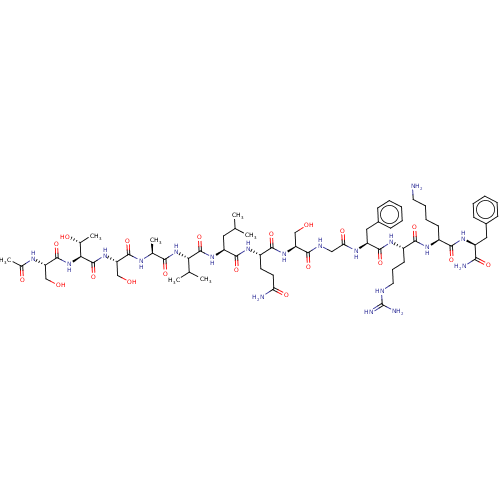
(CHEMBL4875501)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of SARS CoV-2 main protease using varying concentrations of DABCYL-KTSAVLQ1SGFRKM-E(EDANS)-NH2 as substrate by Dixon plot anal... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128333
BindingDB Entry DOI: 10.7270/Q29W0K7J |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
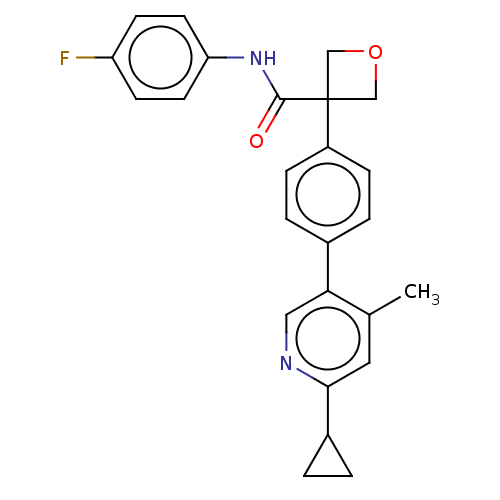
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
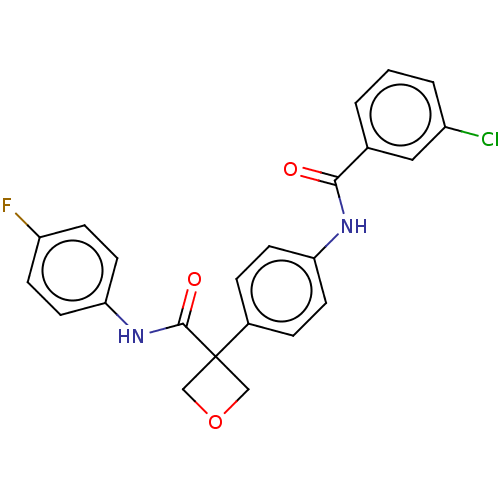
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449372
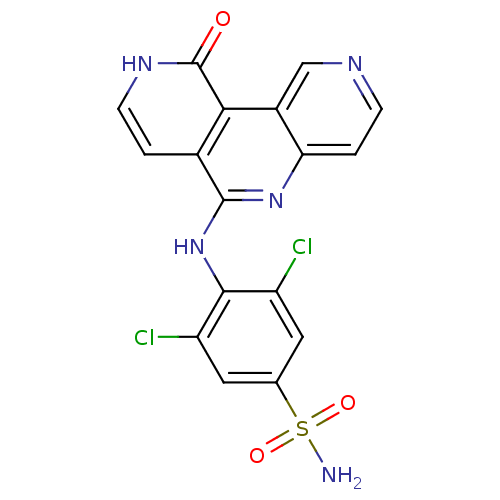
(CHEMBL3126350)Show SMILES NS(=O)(=O)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C17H11Cl2N5O3S/c18-11-5-8(28(20,26)27)6-12(19)15(11)24-16-9-1-4-22-17(25)14(9)10-7-21-3-2-13(10)23-16/h1-7H,(H,22,25)(H,23,24)(H2,20,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
NAD(P)H dehydrogenase [quinone] 1
(Homo sapiens (Human)) | BDBM35536
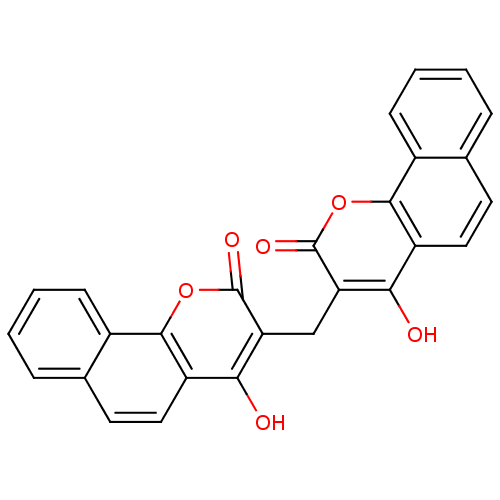
(symmetric dicoumarol analogue, 12)Show SMILES Oc1c(Cc2c(O)c3ccc4ccccc4c3oc2=O)c(=O)oc2c1ccc1ccccc21 Show InChI InChI=1S/C27H16O6/c28-22-18-11-9-14-5-1-3-7-16(14)24(18)32-26(30)20(22)13-21-23(29)19-12-10-15-6-2-4-8-17(15)25(19)33-27(21)31/h1-12,28-29H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Manchester
| Assay Description
Recombinant human NQO1 was obtained from Sigma and diluted in 50 mM phosphate buffer to give an absorbance of 0.1 at 550 nm; 5 uL of this solution wa... |
J Med Chem 52: 7142-56 (2009)
Article DOI: 10.1021/jm9011609
BindingDB Entry DOI: 10.7270/Q2222S40 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50449373

(CHEMBL3126349)Show SMILES OC(C(F)F)c1cc(Cl)c(Nc2nc3ccncc3c3c2cc[nH]c3=O)c(Cl)c1 Show InChI InChI=1S/C19H12Cl2F2N4O2/c20-11-5-8(16(28)17(22)23)6-12(21)15(11)27-18-9-1-4-25-19(29)14(9)10-7-24-3-2-13(10)26-18/h1-7,16-17,28H,(H,25,29)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Jak2 (unknown origin) using biotinylated synthetic peptide substrate by homogeneous time resolved fluorescence assay |
Bioorg Med Chem Lett 24: 1466-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.011
BindingDB Entry DOI: 10.7270/Q2D22034 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data