

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184539 ((2S)-2-(6-butyl-2-(4-(4-(trifluoromethyl)phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184530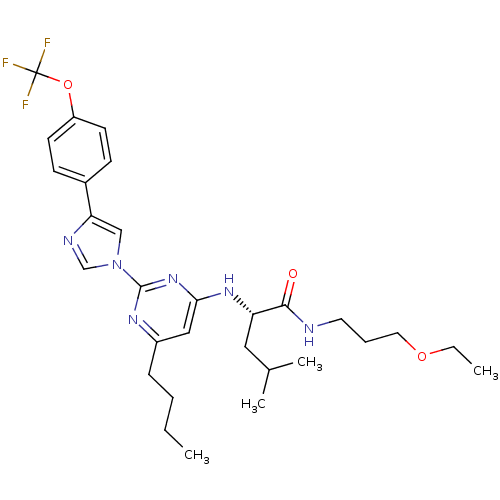 ((2S)-2-(6-butyl-2-(4-(4-(trifluoromethoxy)phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184531 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184518 ((2S)-2-(6-butyl-2-(4-(4-chlorophenyl)-1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184527 ((2S)-2-(6-butyl-2-(4-(4-hydroxyphenyl)-1H-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184540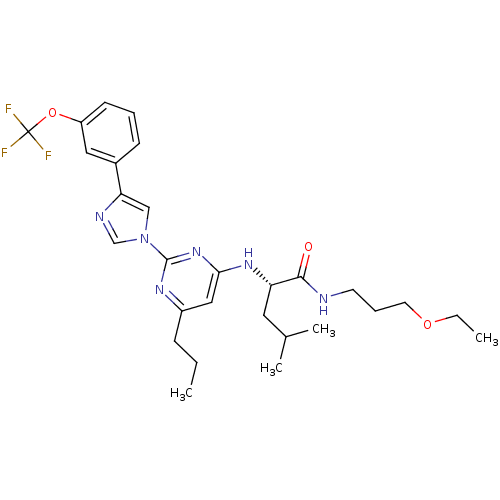 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184536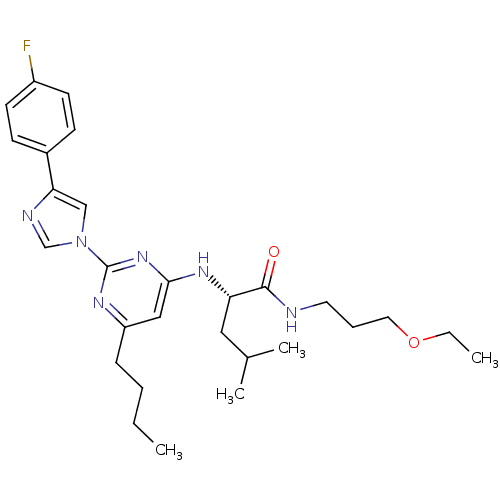 ((2S)-2-(6-butyl-2-(4-(4-fluorophenyl)-1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184535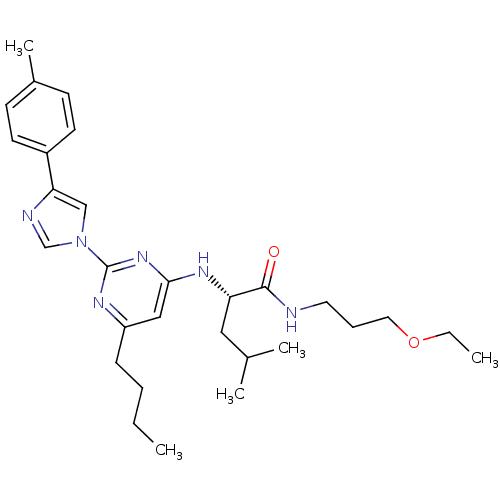 ((2S)-2-(6-butyl-2-(4-p-tolyl-1H-imidazol-1-yl)pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184521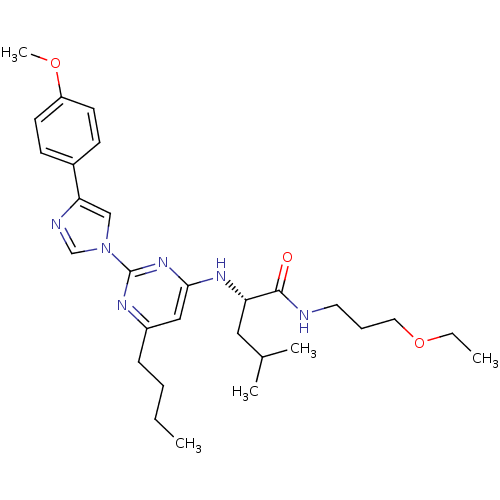 ((2S)-2-(6-butyl-2-(4-(4-methoxyphenyl)-1H-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184523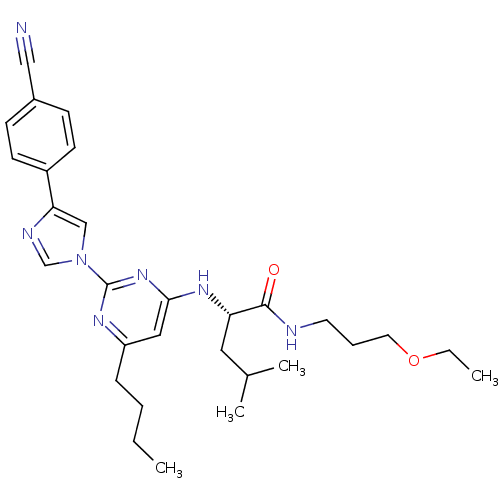 ((2S)-2-(6-butyl-2-(4-(4-cyanophenyl)-1H-imidazol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184517 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-methyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184537 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184516 ((2S)-N-(3-ethoxypropyl)-2-(6-ethyl-2-(4-(4-(triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184524 ((2S)-2-(2-(1H-imidazol-1-yl)-6-(octylthio)pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184532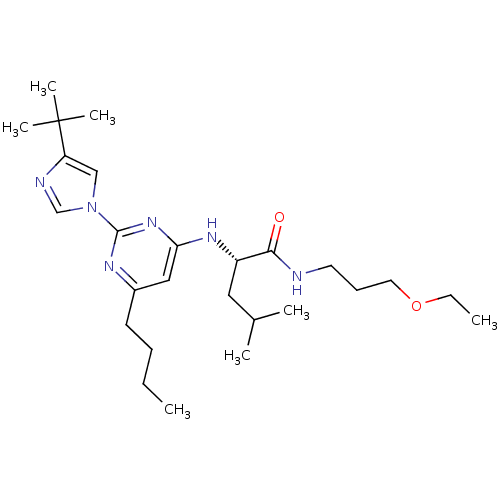 ((2S)-2-(6-butyl-2-(4-tert-butyl-1H-imidazol-1-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184541 ((2S)-2-(6-butyl-2-(4-methyl-1H-imidazol-1-yl)pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184542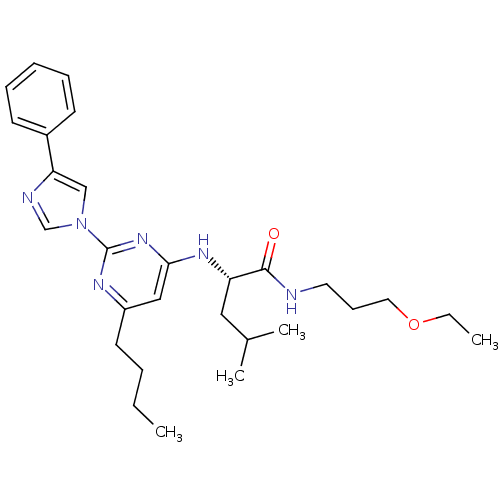 ((2S)-2-(6-butyl-2-(4-phenyl-1H-imidazol-1-yl)pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184519 ((2S)-2-(2-(1H-imidazol-1-yl)-6-octylpyrimidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184526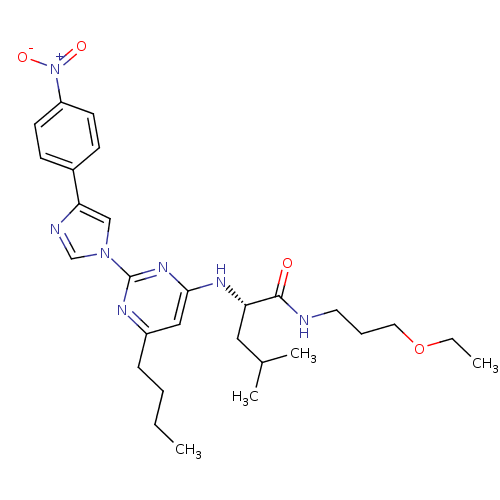 ((2S)-2-(6-butyl-2-(4-(4-nitrophenyl)-1H-imidazol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184529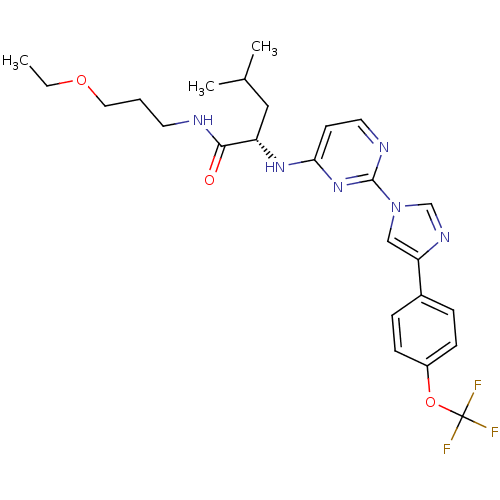 ((2S)-N-(3-ethoxypropyl)-4-methyl-2-(2-(4-(4-(trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184538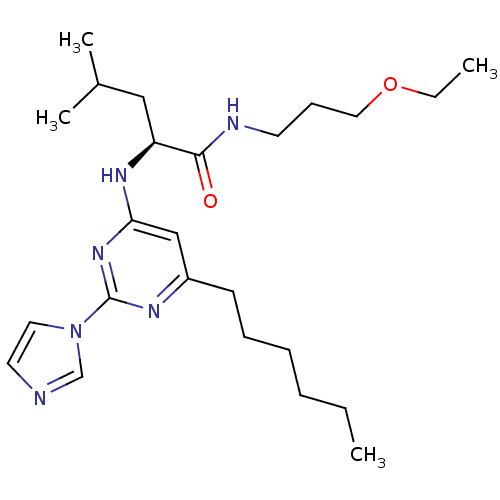 ((2S)-N-(3-ethoxypropyl)-2-(6-hexyl-2-(1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184528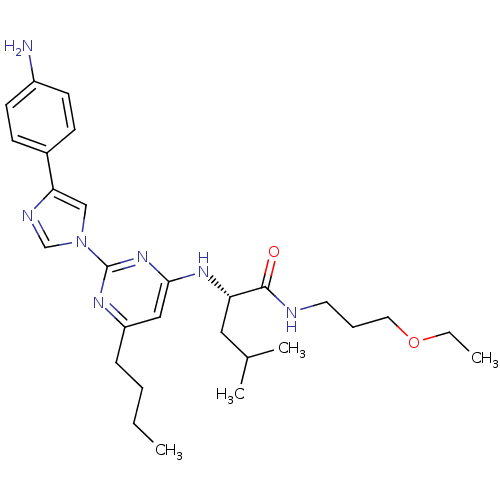 ((2S)-2-(2-(4-(4-aminophenyl)-1H-imidazol-1-yl)-6-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184525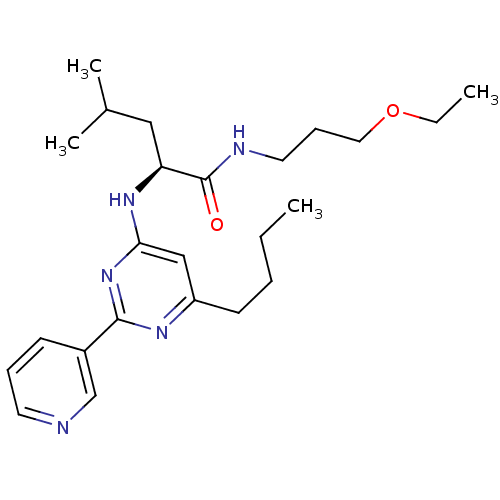 ((2S)-2-(6-butyl-2-(pyridin-3-yl)pyrimidin-4-ylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184533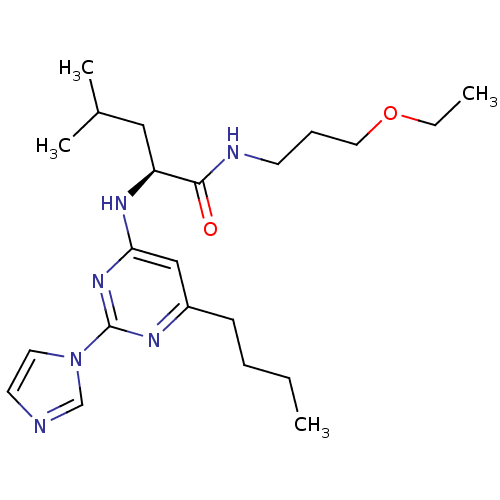 ((2S)-2-(6-butyl-2-(1H-imidazol-1-yl)pyrimidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184515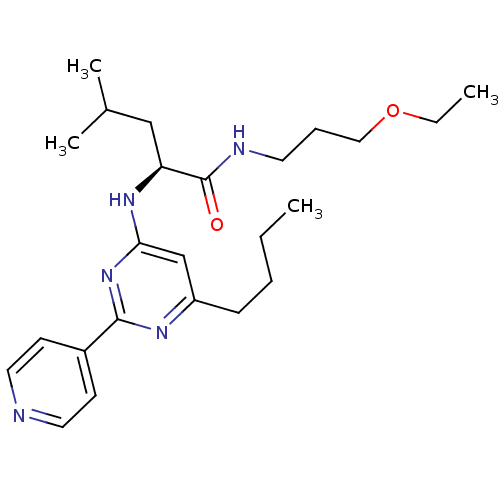 ((S)-2-(6-butyl-2-(pyridin-4-yl)pyrimidin-4-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184534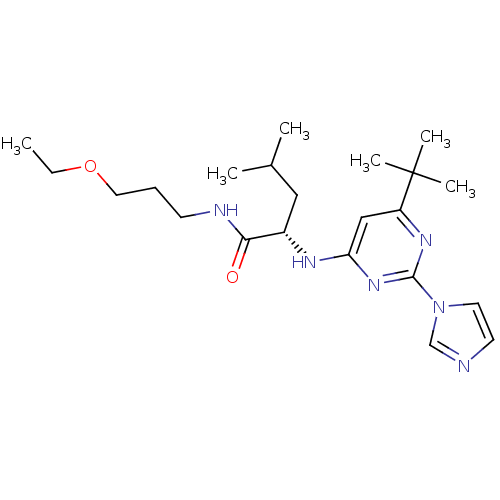 ((2S)-2-(6-tert-butyl-2-(1H-imidazol-1-yl)pyrimidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184522 ((2S)-2-(6-butyl-2-(2-methyl-1H-imidazol-1-yl)pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50184520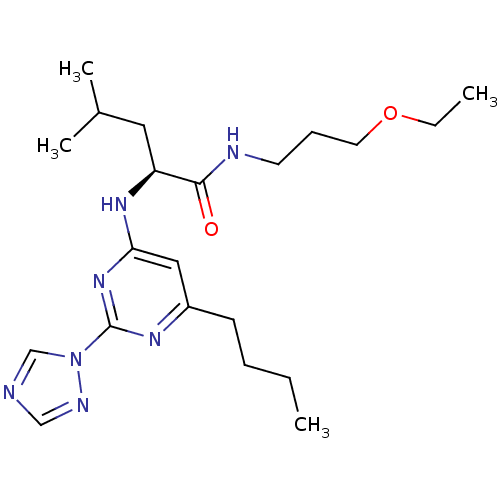 ((2S)-2-(6-butyl-2-(1H-1,2,4-triazol-1-yl)pyrimidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Binding affinity to human CXCR2 receptor transfected in CHO cell | Bioorg Med Chem Lett 16: 2724-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.028 BindingDB Entry DOI: 10.7270/Q2KK9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267904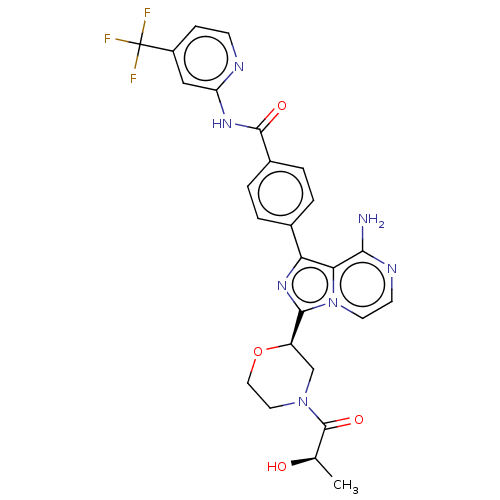 (4-(8-amino-3-{(2R)-4-[(2S)-2-hydroxypropanoyl]morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267905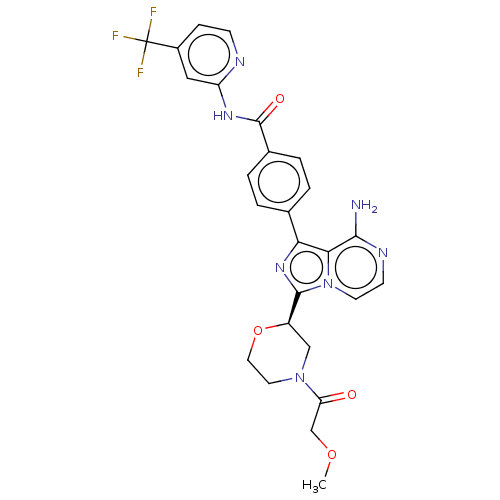 (4-(8-amino-3-{(2R)-4-[(2R)-2-hydroxypropanoyl]morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267906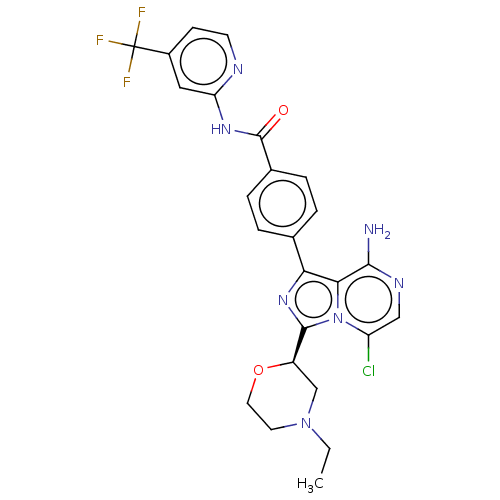 (4-{8-amino-3-[(2R)-4-(methoxyacetyl)morpholin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267907 (4-{8-amino-5-chloro-3-[(2R)-4-ethylmorpholin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267908 (4-(8-amino-5-chloro-3-{(2R)-4-[(2S)-2-hydroxypropa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267909 (4-(8-amino-5-chloro-3-{(2R)-4-[(2R)-2-hydroxypropa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267910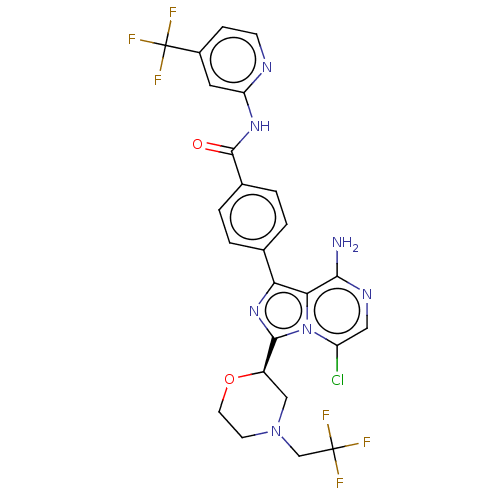 (4-(8-amino-5-chloro-3-{(2R)-4-[(1-hydroxycycloprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267911 (4-{8-amino-5-chloro-3-[(2R)-4-(2,2,2-trifluoroethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267912 (4-(8-amino-5-cyano-3-{(2R)-4-[(3-methyloxetan-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267913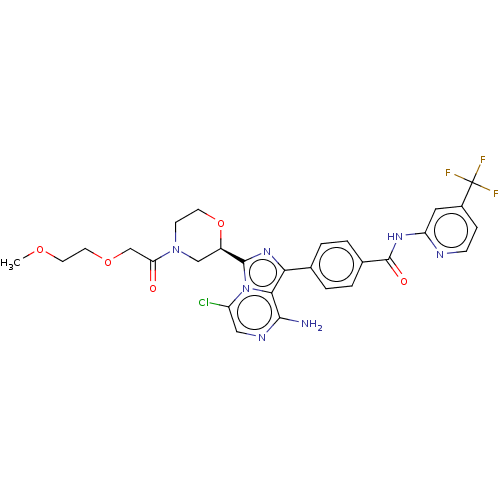 (4-{8-amino-5-chloro-3-[(2R)-4-(methoxyacetyl)morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267915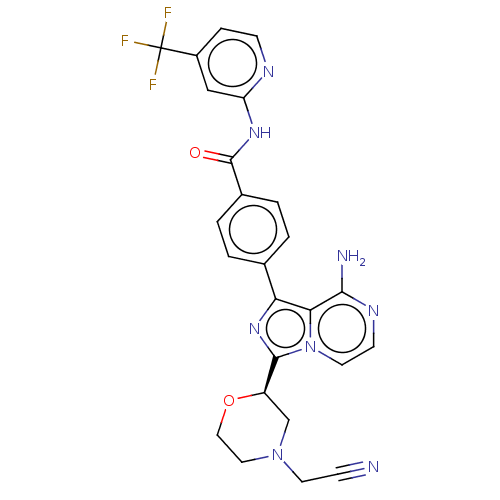 (4-(8-amino-3-{(2S)-4-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267916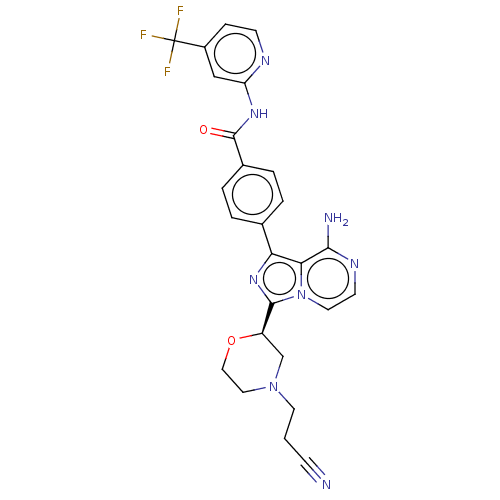 (4-{8-amino-3-[(2R)-4-(cyanomethyl)morpholin-2-yl]i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267917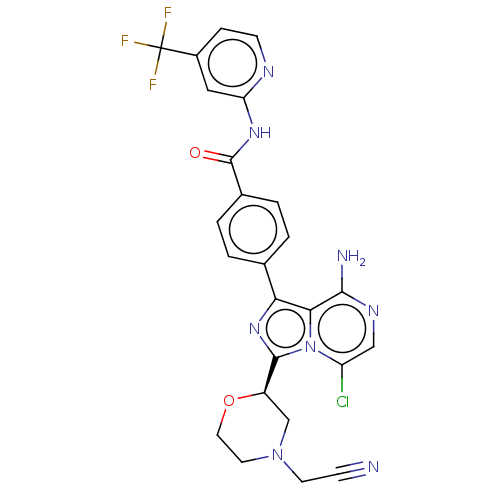 (4-{8-amino-3-[(2R)-4-(2-cyanoethyl)morpholin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267919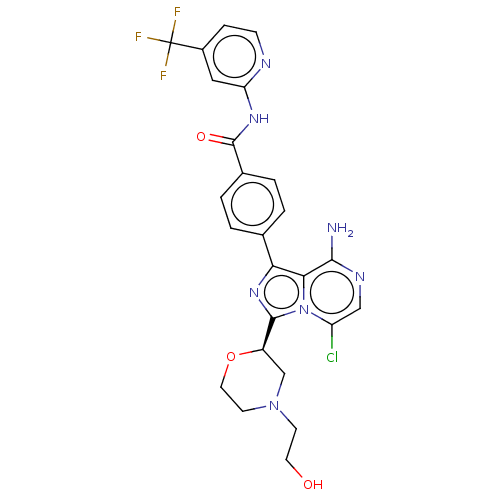 (4-{8-amino-3-[(2S)-4-ethylmorpholin-2-yl]imidazo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267920 (4-{8-amino-5-chloro-3-[(2R)-4-(2-hydroxyethyl)morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267921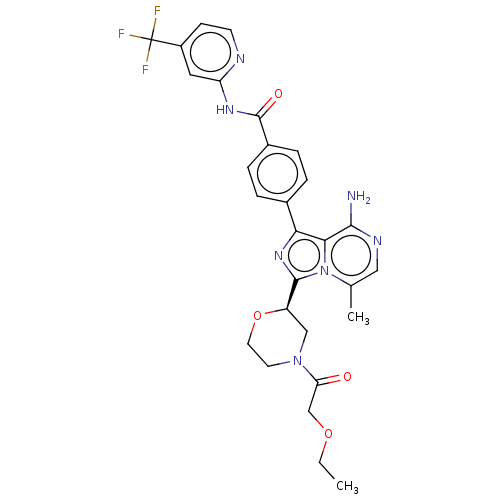 (4-{8-amino-3-[(2R)-4-(methoxyacetyl)morpholin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267922 (4-{8-amino-3-[(2R)-4-(ethoxyacetyl)morpholin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267923 (4-{8-amino-3-[(2R)-4-(difluoroacetyl)morpholin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267924 (4-{8-amino-3-[(2R)-4-(2-methoxyethyl)morpholin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267925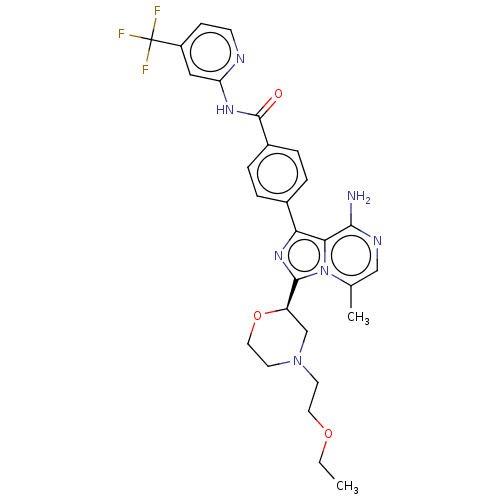 (4-{8-amino-3-[(2R)-4-(2-hydroxyethyl)morpholin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267926 (4-{8-amino-3-[(2R)-4-(2-ethoxyethyl)morpholin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267927 (4-{8-amino-5-chloro-3-[(2R)-4-(2-methoxyethyl)morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2511 total ) | Next | Last >> |