

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178926 (9-cyclopropyl-6-fluoro-7-(piperazin-1-yl)isothiazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178928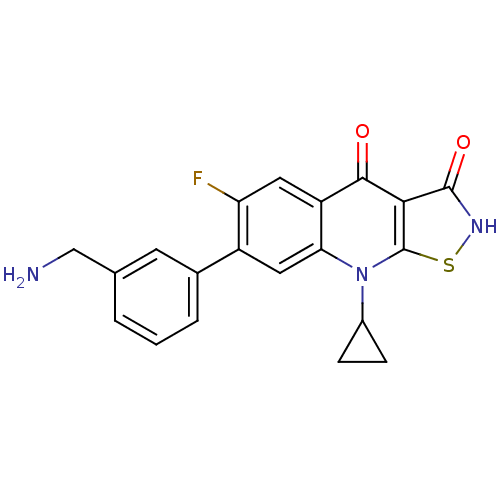 (7-(3-(aminomethyl)phenyl)-9-cyclopropyl-6-fluorois...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178922 (7-(4-(aminomethyl)phenyl)-9-cyclopropyl-6-fluorois...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50180009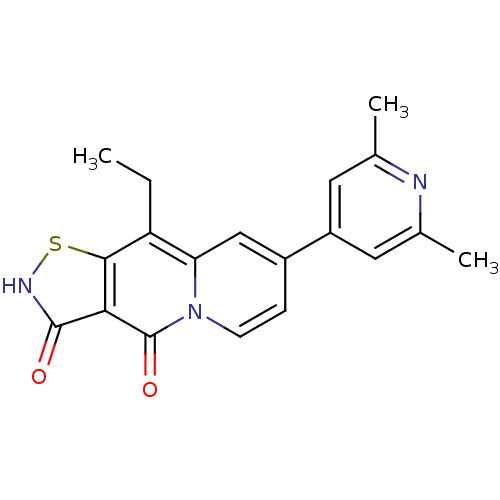 (7-(2,6-dimethylpyridin-4-yl)-9-ethyl-1-thia-2,4a-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DNA gyrase supercoiling in Escherichia coli ATCC 25922 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178928 (7-(3-(aminomethyl)phenyl)-9-cyclopropyl-6-fluorois...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178924 (9-cyclopropyl-6-fluoro-7-(3-hydroxyphenyl)isothiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DNA gyrase supercoiling in Escherichia coli ATCC 25922 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50131428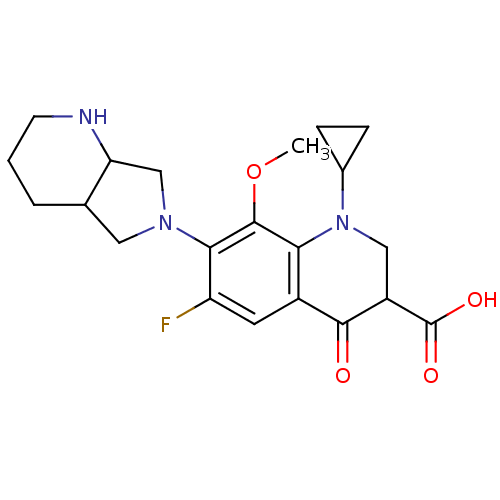 (1-Cyclopropyl-6-fluoro-8-methoxy-7-(1S,7aS)-octahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178923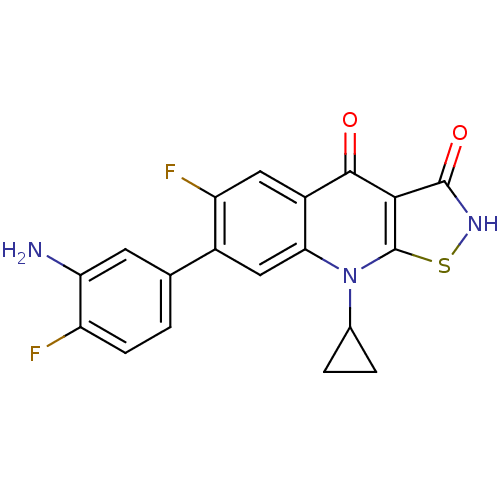 (7-(3-amino-4-fluorophenyl)-9-cyclopropyl-6-fluoroi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178919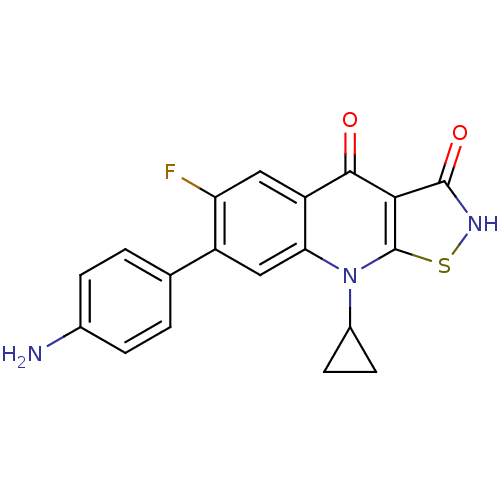 (7-(4-aminophenyl)-9-cyclopropyl-6-fluoroisothiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178917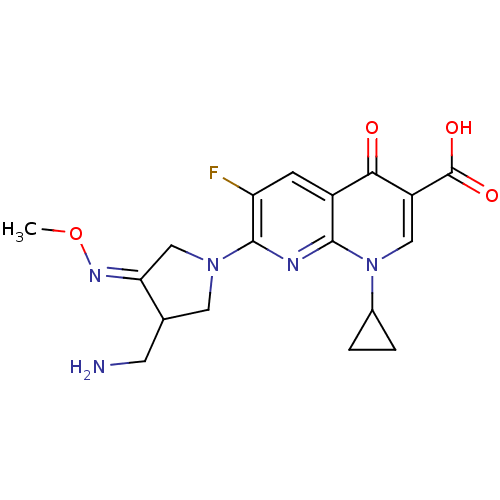 ((S,Z)-7-(3-(aminomethyl)-4-(methoxyimino)pyrrolidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50180011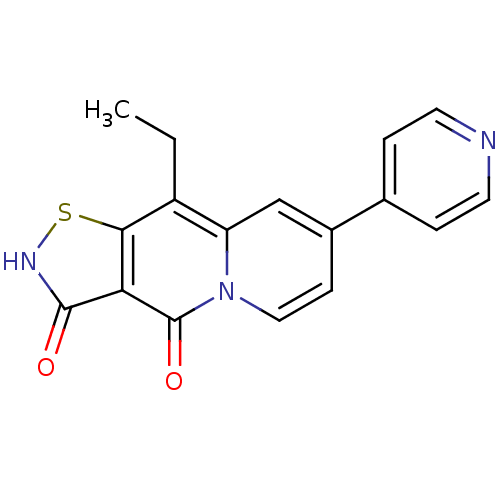 (9-ethyl-7-pyridin-4-yl-1-thia-2,4a-diaza-cyclopent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DNA gyrase supercoiling in Escherichia coli ATCC 25922 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50117914 (1-Cyclopropyl-1,4-dihydro-6-fluoro-8-methoxy-7-(3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178926 (9-cyclopropyl-6-fluoro-7-(piperazin-1-yl)isothiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178918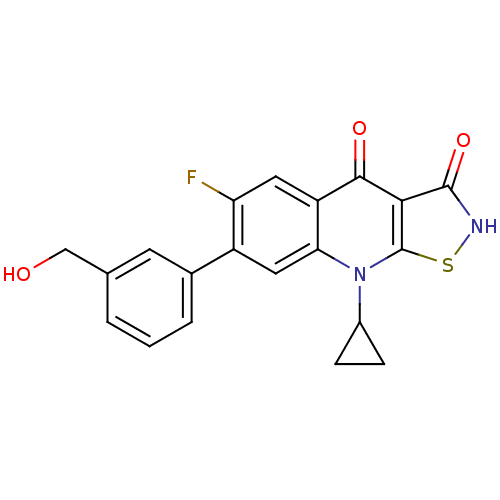 (9-cyclopropyl-6-fluoro-7-(3-(hydroxymethyl)phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178912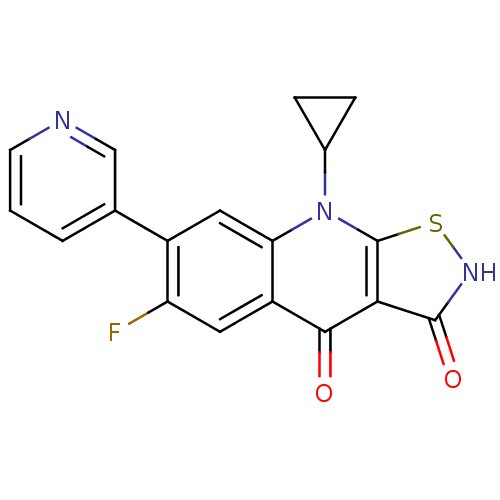 (9-cyclopropyl-6-fluoro-7-(pyridin-3-yl)isothiazolo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178917 ((S,Z)-7-(3-(aminomethyl)-4-(methoxyimino)pyrrolidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50045000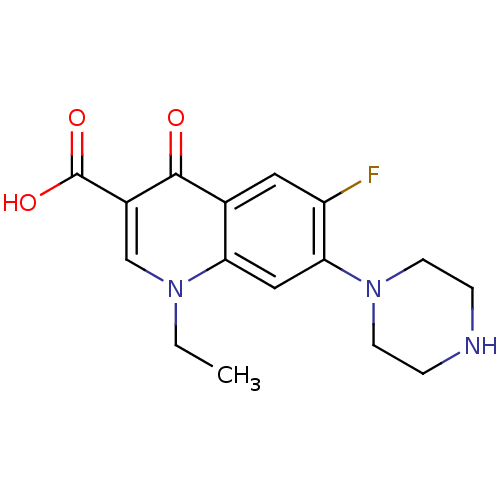 ((NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DNA gyrase supercoiling in Escherichia coli ATCC 25922 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50330327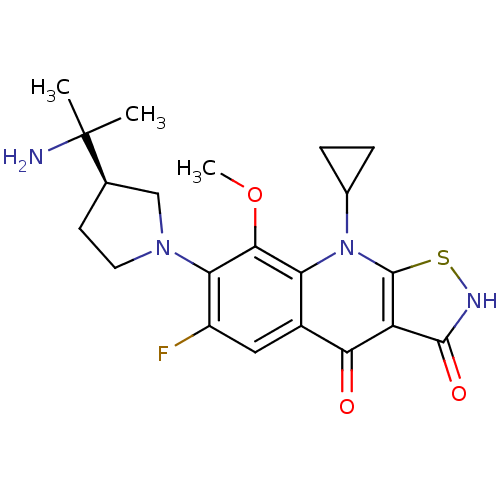 ((R)-7-(3-(2-aminopropan-2-yl)pyrrolidin-1-yl)-9-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type Staphylococcus aureus DNA gyrase assessed as inhibition of supercoiling of pBR322 DNA after 60 min by gel electrophoresis ass... | J Med Chem 54: 3268-82 (2011) Article DOI: 10.1021/jm101604v BindingDB Entry DOI: 10.7270/Q28S4SRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50483850 (CHEMBL1774147) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type Staphylococcus aureus DNA gyrase assessed as inhibition of supercoiling of pBR322 DNA after 60 min by gel electrophoresis ass... | J Med Chem 54: 3268-82 (2011) Article DOI: 10.1021/jm101604v BindingDB Entry DOI: 10.7270/Q28S4SRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178913 (7-(6-(aminomethyl)pyridin-3-yl)-9-cyclopropyl-6-fl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178925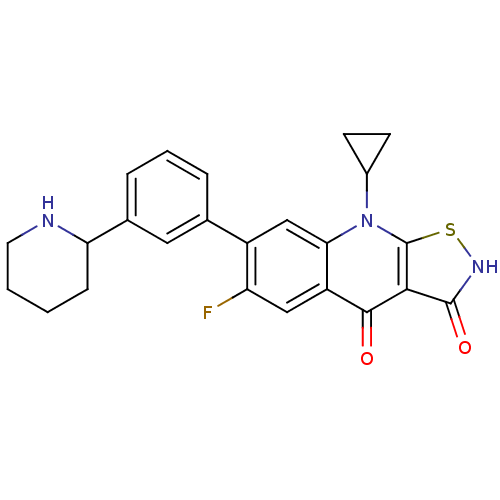 (9-cyclopropyl-6-fluoro-7-(3-(piperidin-2-yl)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50131428 (1-Cyclopropyl-6-fluoro-8-methoxy-7-(1S,7aS)-octahy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50131428 (1-Cyclopropyl-6-fluoro-8-methoxy-7-(1S,7aS)-octahy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178921 (9-cyclopropyl-6-fluoro-7-(4-hydroxyphenyl)isothiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50178916 (9-cyclopropyl-6-fluoro-7-(4-(hydroxymethyl)phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Escherichia coli gyrase | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109055 (US9695205, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109056 (US9695205, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109057 (US9695205, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109058 (US9695205, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109059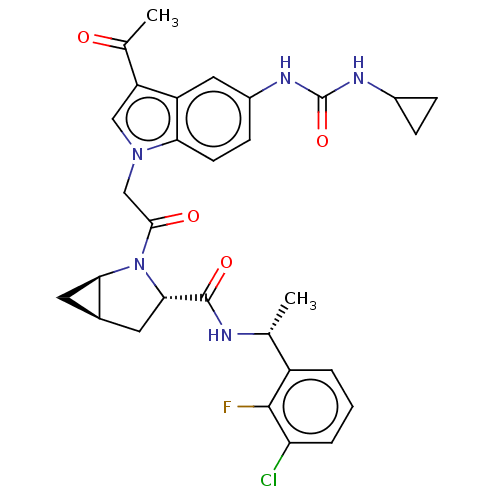 (US9695205, 5) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109061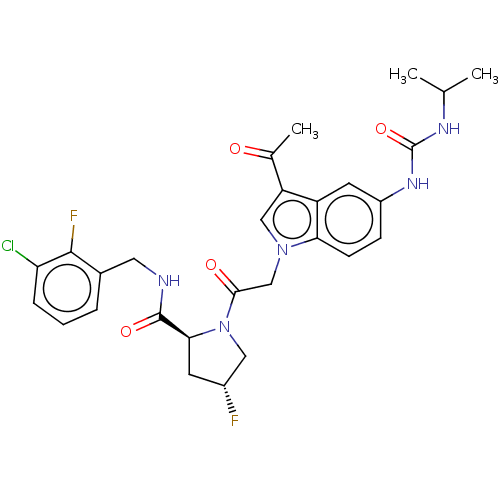 (US9695205, 6) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109091 (US9695205, 7) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109092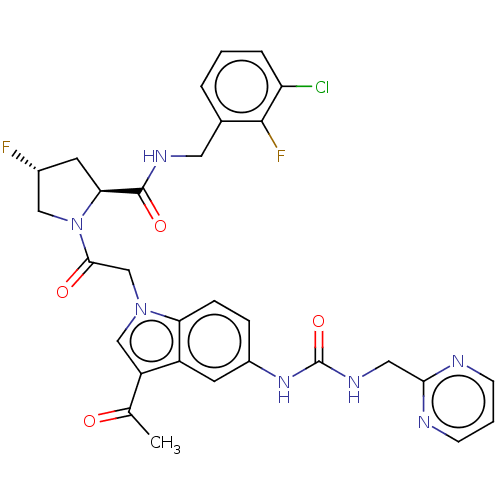 (US9695205, 8) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109093 (US9695205, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109094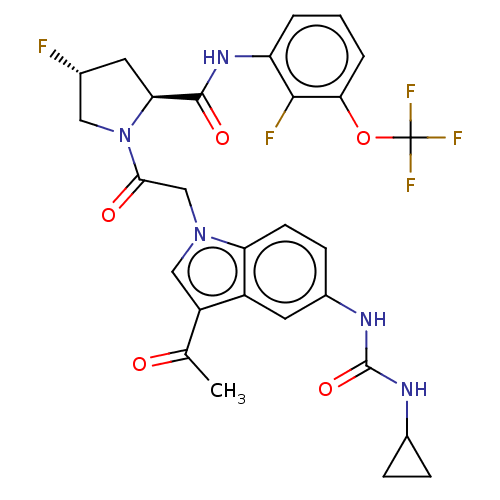 (US9695205, 10) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109103 (US9695205, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109104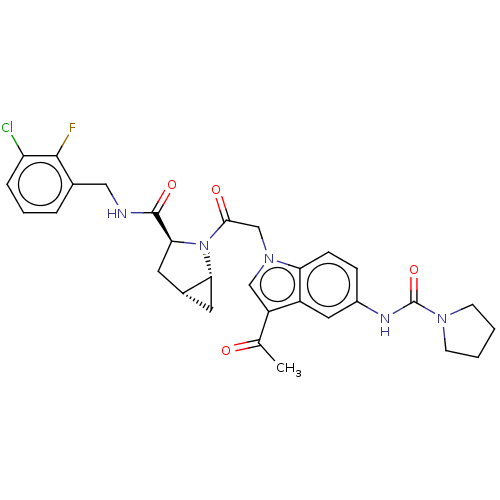 (US9695205, 12) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109178 (US9695205, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109199 (US9695205, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109358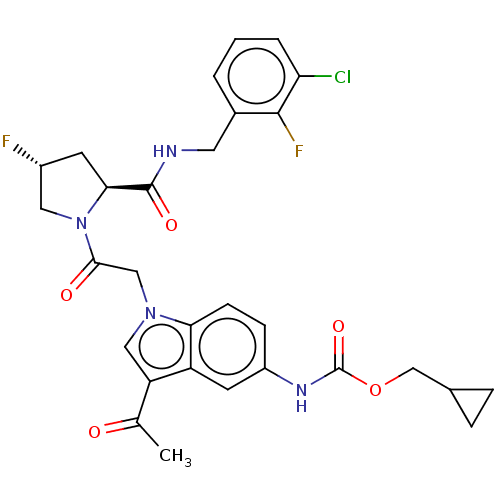 (US9695205, 15) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109385 (US9695205, 16) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109391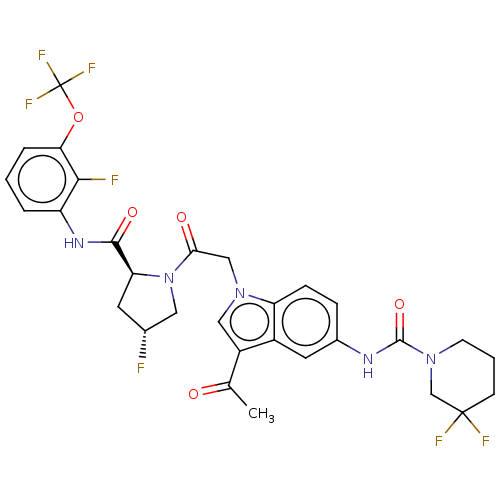 (US9695205, 17) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109393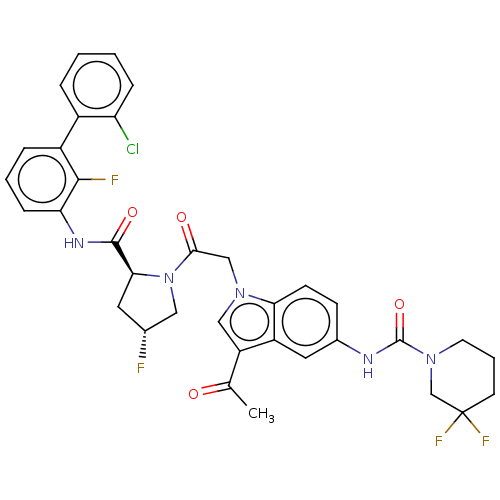 (US9695205, 18) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109466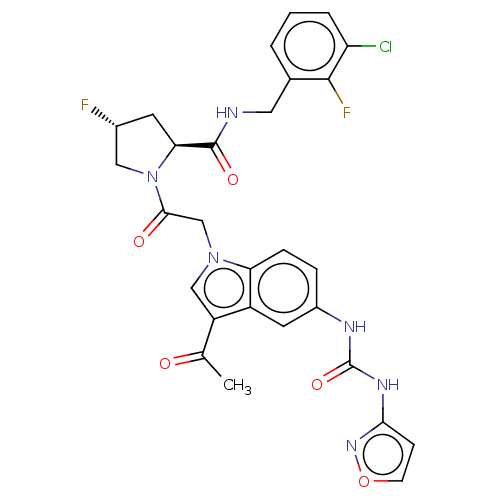 (US9695205, 19) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109468 (US9695205, 20) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109469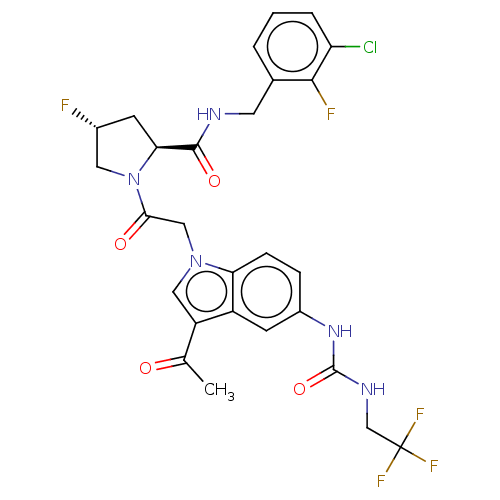 (US9695205, 21) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109544 (US9695205, 22) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109545 (US9695205, 23) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4222 total ) | Next | Last >> |