 Found 125 hits with Last Name = 'wilkinson' and Initial = 'v'
Found 125 hits with Last Name = 'wilkinson' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50051653
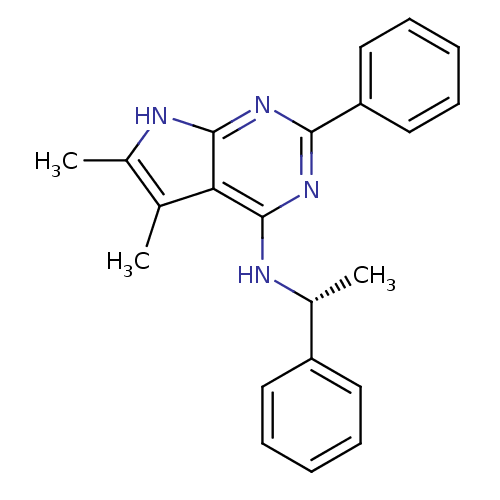
((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show SMILES C[C@@H](Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H22N4/c1-14-15(2)23-21-19(14)22(24-16(3)17-10-6-4-7-11-17)26-20(25-21)18-12-8-5-9-13-18/h4-13,16H,1-3H3,(H2,23,24,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity towards rat A1 receptor was determined |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080288
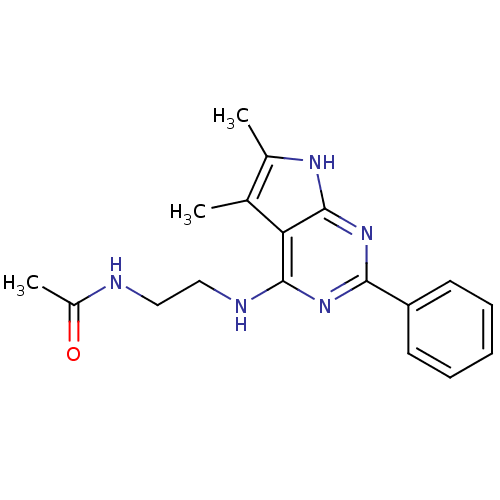
(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080288

(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080292
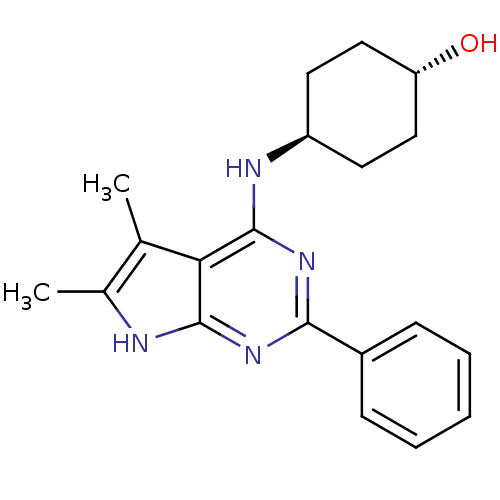
(4-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidi...)Show SMILES Cc1[nH]c2nc(nc(N[C@H]3CC[C@H](O)CC3)c2c1C)-c1ccccc1 |wU:9.8,wD:12.12,(7.76,-9.44,;6.22,-9.46,;5.3,-10.73,;3.85,-10.24,;2.5,-11.04,;1.17,-10.27,;1.17,-8.73,;2.5,-7.93,;2.48,-6.39,;1.15,-5.65,;-.16,-6.42,;-1.5,-5.65,;-1.5,-4.11,;-2.84,-3.34,;-.16,-3.34,;1.17,-4.09,;3.83,-8.7,;5.3,-8.22,;5.75,-6.75,;-.16,-11.04,;-.15,-12.55,;-1.5,-13.32,;-2.82,-12.55,;-2.82,-11.01,;-1.5,-10.24,)| Show InChI InChI=1S/C20H24N4O/c1-12-13(2)21-19-17(12)20(22-15-8-10-16(25)11-9-15)24-18(23-19)14-6-4-3-5-7-14/h3-7,15-16,25H,8-11H2,1-2H3,(H2,21,22,23,24)/t15-,16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080291
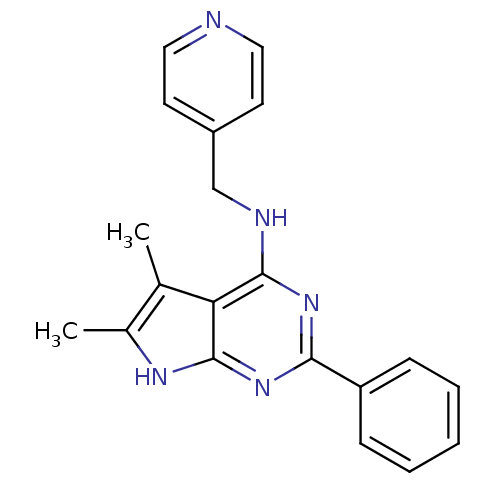
((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show InChI InChI=1S/C20H19N5/c1-13-14(2)23-20-17(13)19(22-12-15-8-10-21-11-9-15)24-18(25-20)16-6-4-3-5-7-16/h3-11H,12H2,1-2H3,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080287
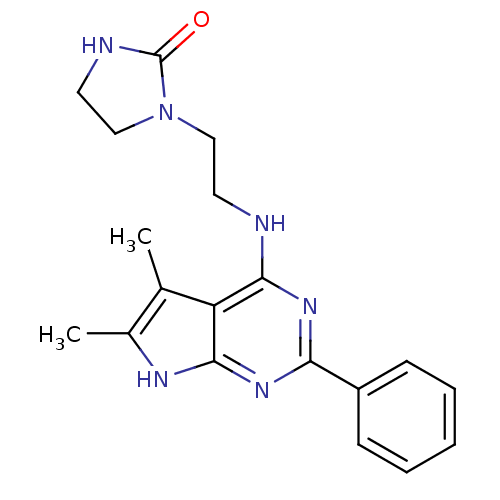
(1-[2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrim...)Show SMILES Cc1[nH]c2nc(nc(NCCN3CCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C19H22N6O/c1-12-13(2)22-18-15(12)17(20-8-10-25-11-9-21-19(25)26)23-16(24-18)14-6-4-3-5-7-14/h3-7H,8-11H2,1-2H3,(H,21,26)(H2,20,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080287

(1-[2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrim...)Show SMILES Cc1[nH]c2nc(nc(NCCN3CCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C19H22N6O/c1-12-13(2)22-18-15(12)17(20-8-10-25-11-9-21-19(25)26)23-16(24-18)14-6-4-3-5-7-14/h3-7H,8-11H2,1-2H3,(H,21,26)(H2,20,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080292

(4-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidi...)Show SMILES Cc1[nH]c2nc(nc(N[C@H]3CC[C@H](O)CC3)c2c1C)-c1ccccc1 |wU:9.8,wD:12.12,(7.76,-9.44,;6.22,-9.46,;5.3,-10.73,;3.85,-10.24,;2.5,-11.04,;1.17,-10.27,;1.17,-8.73,;2.5,-7.93,;2.48,-6.39,;1.15,-5.65,;-.16,-6.42,;-1.5,-5.65,;-1.5,-4.11,;-2.84,-3.34,;-.16,-3.34,;1.17,-4.09,;3.83,-8.7,;5.3,-8.22,;5.75,-6.75,;-.16,-11.04,;-.15,-12.55,;-1.5,-13.32,;-2.82,-12.55,;-2.82,-11.01,;-1.5,-10.24,)| Show InChI InChI=1S/C20H24N4O/c1-12-13(2)21-19-17(12)20(22-15-8-10-16(25)11-9-15)24-18(23-19)14-6-4-3-5-7-14/h3-7,15-16,25H,8-11H2,1-2H3,(H2,21,22,23,24)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 754 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-CGS-21,680 to human A2a receptor (hA2a) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080289
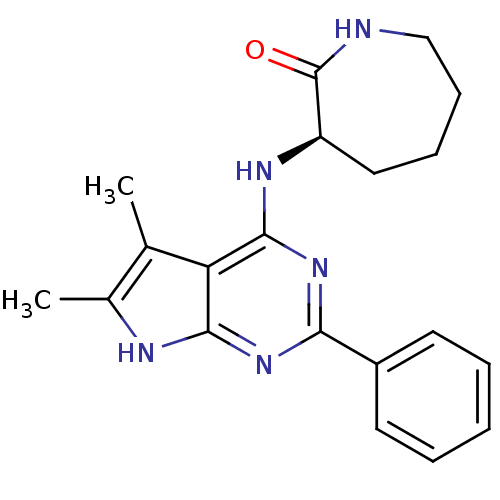
((R)-3-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES Cc1[nH]c2nc(nc(N[C@@H]3CCCCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C20H23N5O/c1-12-13(2)22-18-16(12)19(23-15-10-6-7-11-21-20(15)26)25-17(24-18)14-8-4-3-5-9-14/h3-5,8-9,15H,6-7,10-11H2,1-2H3,(H,21,26)(H2,22,23,24,25)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 771 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080291

((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show InChI InChI=1S/C20H19N5/c1-13-14(2)23-20-17(13)19(22-12-15-8-10-21-11-9-15)24-18(25-20)16-6-4-3-5-7-16/h3-11H,12H2,1-2H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 811 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080290

((1R,2R)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]...)Show SMILES Cc1[nH]c2nc(nc(N[C@@H]3Cc4ccccc4[C@H]3O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C23H22N4O/c1-13-14(2)24-22-19(13)23(27-21(26-22)15-8-4-3-5-9-15)25-18-12-16-10-6-7-11-17(16)20(18)28/h3-11,18,20,28H,12H2,1-2H3,(H2,24,25,26,27)/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 896 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080286
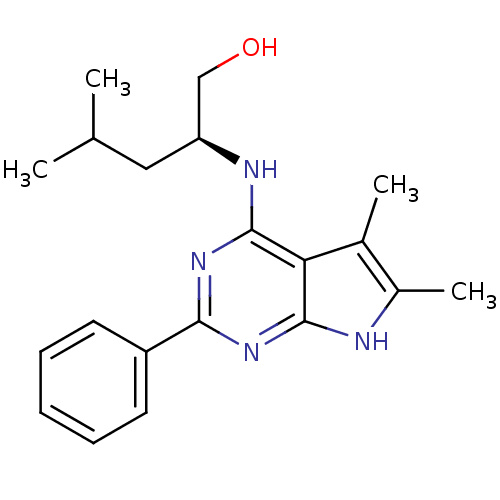
((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CC(C)C[C@@H](CO)Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C20H26N4O/c1-12(2)10-16(11-25)22-20-17-13(3)14(4)21-19(17)23-18(24-20)15-8-6-5-7-9-15/h5-9,12,16,25H,10-11H2,1-4H3,(H2,21,22,23,24)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 951 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080289

((R)-3-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES Cc1[nH]c2nc(nc(N[C@@H]3CCCCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C20H23N5O/c1-12-13(2)22-18-16(12)19(23-15-10-6-7-11-21-20(15)26)25-17(24-18)14-8-4-3-5-9-14/h3-5,8-9,15H,6-7,10-11H2,1-2H3,(H,21,26)(H2,22,23,24,25)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50051653

((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show SMILES C[C@@H](Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H22N4/c1-14-15(2)23-21-19(14)22(24-16(3)17-10-6-4-7-11-17)26-20(25-21)18-12-8-5-9-13-18/h4-13,16H,1-3H3,(H2,23,24,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 981 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080286

((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CC(C)C[C@@H](CO)Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C20H26N4O/c1-12(2)10-16(11-25)22-20-17-13(3)14(4)21-19(17)23-18(24-20)15-8-6-5-7-9-15/h5-9,12,16,25H,10-11H2,1-4H3,(H2,21,22,23,24)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552190

(CHEMBL4741714)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccccc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,1.0,3.3,(34.39,-20.74,;33.06,-21.51,;31.73,-20.73,;30.39,-21.51,;29.06,-20.73,;30.39,-23.05,;31.72,-23.81,;33.06,-23.04,;31.73,-25.34,;30.4,-26.11,;30.4,-27.66,;31.73,-28.42,;33.07,-27.65,;33.07,-26.11,;34.4,-25.33,;35.74,-26.1,;35.74,-27.63,;37.07,-25.32,;38.4,-26.09,;39.72,-25.31,;39.72,-23.77,;41.05,-23,;38.38,-23.01,;37.05,-23.79,;38.36,-21.48,;39.69,-20.7,;41.03,-21.46,;39.68,-19.16,;38.34,-18.4,;37.02,-19.19,;37.03,-20.72,;35.71,-21.51,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50552190

(CHEMBL4741714)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccccc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,1.0,3.3,(34.39,-20.74,;33.06,-21.51,;31.73,-20.73,;30.39,-21.51,;29.06,-20.73,;30.39,-23.05,;31.72,-23.81,;33.06,-23.04,;31.73,-25.34,;30.4,-26.11,;30.4,-27.66,;31.73,-28.42,;33.07,-27.65,;33.07,-26.11,;34.4,-25.33,;35.74,-26.1,;35.74,-27.63,;37.07,-25.32,;38.4,-26.09,;39.72,-25.31,;39.72,-23.77,;41.05,-23,;38.38,-23.01,;37.05,-23.79,;38.36,-21.48,;39.69,-20.7,;41.03,-21.46,;39.68,-19.16,;38.34,-18.4,;37.02,-19.19,;37.03,-20.72,;35.71,-21.51,)| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50552190

(CHEMBL4741714)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccccc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,1.0,3.3,(34.39,-20.74,;33.06,-21.51,;31.73,-20.73,;30.39,-21.51,;29.06,-20.73,;30.39,-23.05,;31.72,-23.81,;33.06,-23.04,;31.73,-25.34,;30.4,-26.11,;30.4,-27.66,;31.73,-28.42,;33.07,-27.65,;33.07,-26.11,;34.4,-25.33,;35.74,-26.1,;35.74,-27.63,;37.07,-25.32,;38.4,-26.09,;39.72,-25.31,;39.72,-23.77,;41.05,-23,;38.38,-23.01,;37.05,-23.79,;38.36,-21.48,;39.69,-20.7,;41.03,-21.46,;39.68,-19.16,;38.34,-18.4,;37.02,-19.19,;37.03,-20.72,;35.71,-21.51,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552189
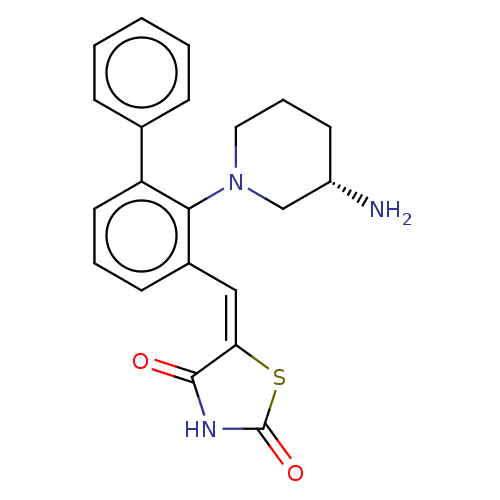
(CHEMBL4793492)Show SMILES N[C@H]1CCCN(C1)c1c(\C=C2/SC(=O)NC2=O)cccc1-c1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PIM1 using full length BAD peptide substrate in presence of ATP at Km concentration |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50552189

(CHEMBL4793492)Show SMILES N[C@H]1CCCN(C1)c1c(\C=C2/SC(=O)NC2=O)cccc1-c1ccccc1 |r| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PIM3 using full length BAD peptide substrate in presence of ATP at Km concentration |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552147
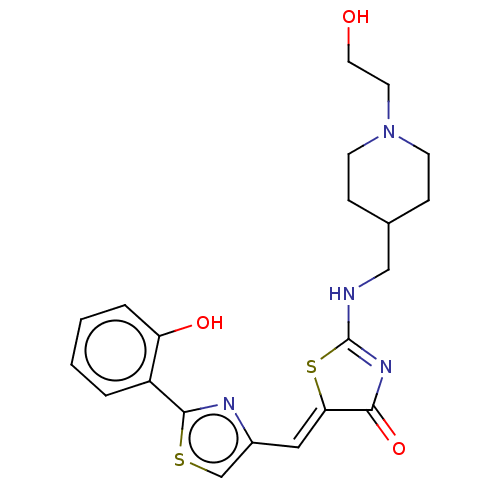
(CHEMBL4794742)Show SMILES OCCN1CCC(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccccc2O)CC1 |t:9| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50552189

(CHEMBL4793492)Show SMILES N[C@H]1CCCN(C1)c1c(\C=C2/SC(=O)NC2=O)cccc1-c1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PIM2 using full length BAD peptide substrate in presence of ATP at Km concentration |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552146

(CHEMBL4747343)Show SMILES OC[C@H]1CCC[C@@H]1NC1=NC(=O)\C(S1)=C\c1csc(n1)-c1cccc(F)c1O |r,t:9| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552149
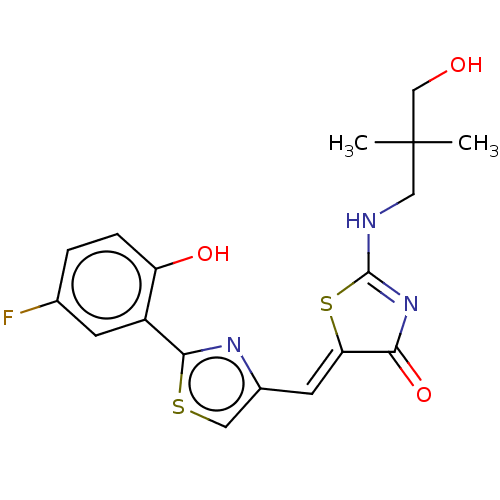
(CHEMBL4778893)Show SMILES CC(C)(CO)CNC1=NC(=O)\C(S1)=C\c1csc(n1)-c1cc(F)ccc1O |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50364776
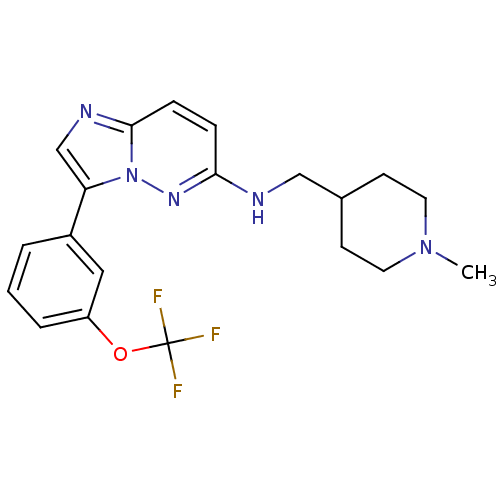
(CHEMBL1952141 | CHEMBL1952329)Show SMILES CN1CCC(CNc2ccc3ncc(-c4cccc(OC(F)(F)F)c4)n3n2)CC1 Show InChI InChI=1S/C20H22F3N5O/c1-27-9-7-14(8-10-27)12-24-18-5-6-19-25-13-17(28(19)26-18)15-3-2-4-16(11-15)29-20(21,22)23/h2-6,11,13-14H,7-10,12H2,1H3,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged PIM1 expressed in Escherichia coli using KKRNRTLTV as substrate incubated for 40 mi... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552171
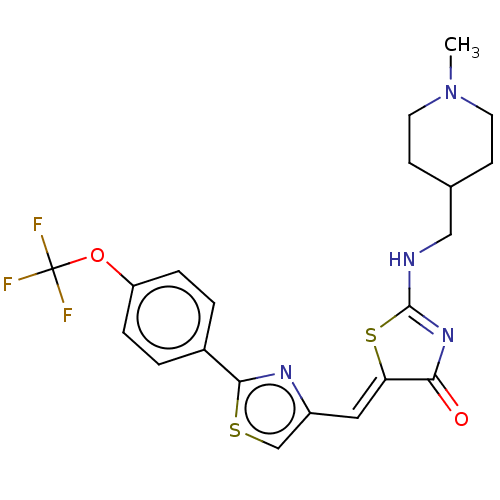
(CHEMBL4757567)Show SMILES CN1CCC(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccc(OC(F)(F)F)cc2)CC1 |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PIM1 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552173
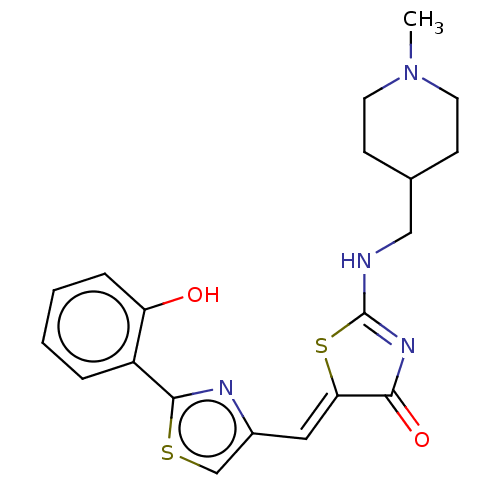
(CHEMBL4788212)Show SMILES CN1CCC(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccccc2O)CC1 |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human A1 receptor (hA1) on membranes from yeast cells |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552150

(CHEMBL4797284)Show SMILES CC(C)[C@@H]1CC[C@@H](CO)[C@H]1NC1=NC(=O)\C(S1)=C\c1csc(n1)-c1ccccc1O |r,t:12| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552153
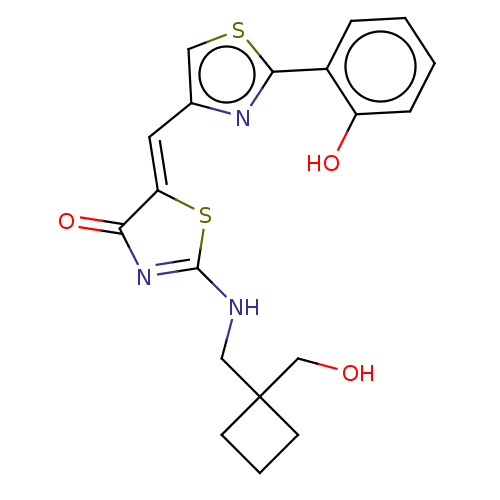
(CHEMBL4794863)Show SMILES OCC1(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccccc2O)CCC1 |t:5| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552155

(CHEMBL4777003)Show SMILES CC(C)(CO)CNC1=NC(=O)\C(S1)=C\c1csc(n1)-c1ccccc1O |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552154
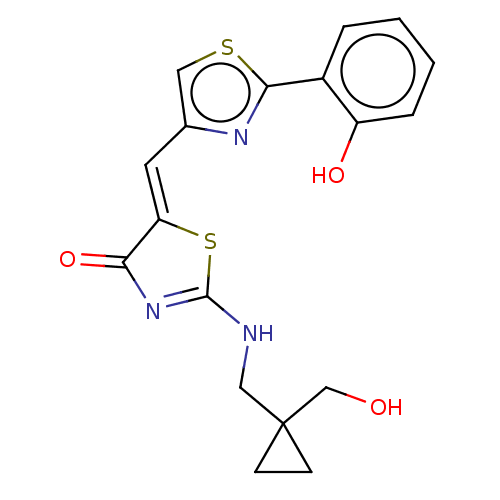
(CHEMBL4798741)Show SMILES OCC1(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccccc2O)CC1 |t:5| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552178
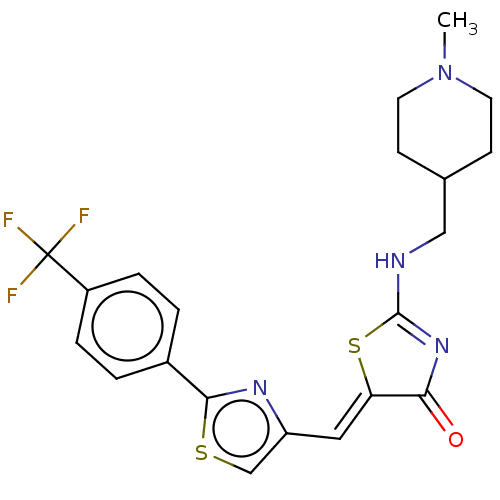
(CHEMBL4758677)Show SMILES CN1CCC(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccc(cc2)C(F)(F)F)CC1 |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552171

(CHEMBL4757567)Show SMILES CN1CCC(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccc(OC(F)(F)F)cc2)CC1 |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552157
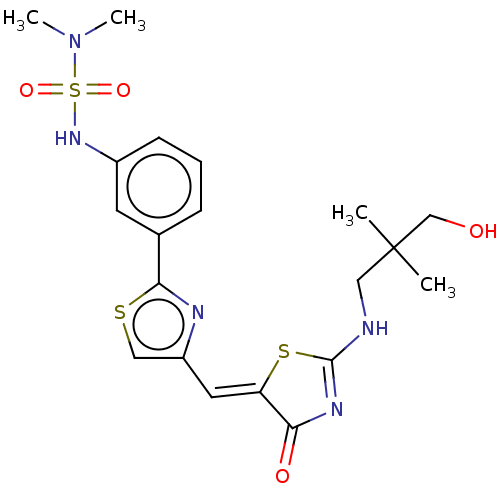
(CHEMBL4786077)Show SMILES CN(C)S(=O)(=O)Nc1cccc(c1)-c1nc(\C=C2/SC(NCC(C)(C)CO)=NC2=O)cs1 |c:27| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552163

(CHEMBL4783270)Show SMILES CN(c1cccc(c1)-c1nc(\C=C2/SC(NCC3CCN(C)CC3)=NC2=O)cs1)S(C)(=O)=O |c:25| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080288

(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against membranes from HEK293 cells stably expressing the human A2a receptor (hA2a) |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080288

(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against membranes from HEK293 cells stably expressing the human A2a receptor (hA2a) |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552152

(CHEMBL4783323)Show SMILES CN1CCC(O)(CNC2=NC(=O)\C(S2)=C\c2csc(n2)-c2ccc(OC(F)(F)F)cc2)CC1 |t:8| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552184
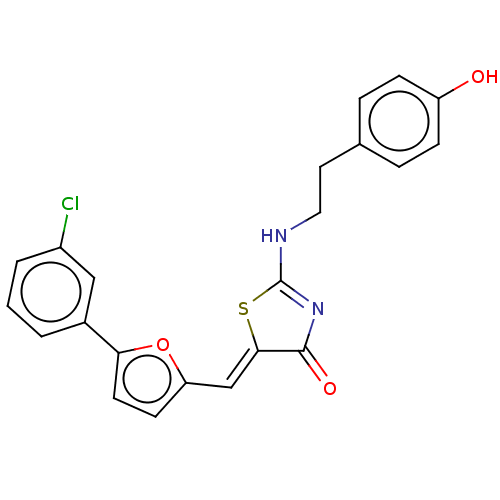
(CHEMBL4751389)Show SMILES Oc1ccc(CCNC2=NC(=O)\C(S2)=C\c2ccc(o2)-c2cccc(Cl)c2)cc1 |t:8| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552185

(CHEMBL4781049)Show SMILES CN1CCC(CNC2=NC(=O)\C(S2)=C\c2ccc(o2)-c2cccc(Cl)c2)CC1 |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552151

(CHEMBL4763088)Show SMILES CC(C)(CO)CNC1=NC(=O)\C(S1)=C\c1csc(n1)-c1cccc(F)c1O |t:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080292

(4-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidi...)Show SMILES Cc1[nH]c2nc(nc(N[C@H]3CC[C@H](O)CC3)c2c1C)-c1ccccc1 |wU:9.8,wD:12.12,(7.76,-9.44,;6.22,-9.46,;5.3,-10.73,;3.85,-10.24,;2.5,-11.04,;1.17,-10.27,;1.17,-8.73,;2.5,-7.93,;2.48,-6.39,;1.15,-5.65,;-.16,-6.42,;-1.5,-5.65,;-1.5,-4.11,;-2.84,-3.34,;-.16,-3.34,;1.17,-4.09,;3.83,-8.7,;5.3,-8.22,;5.75,-6.75,;-.16,-11.04,;-.15,-12.55,;-1.5,-13.32,;-2.82,-12.55,;-2.82,-11.01,;-1.5,-10.24,)| Show InChI InChI=1S/C20H24N4O/c1-12-13(2)21-19-17(12)20(22-15-8-10-16(25)11-9-15)24-18(23-19)14-6-4-3-5-7-14/h3-7,15-16,25H,8-11H2,1-2H3,(H2,21,22,23,24)/t15-,16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50552172
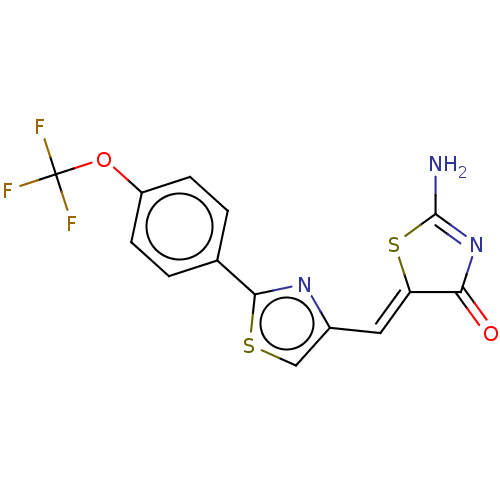
(CHEMBL4742215)Show SMILES NC1=NC(=O)\C(S1)=C\c1csc(n1)-c1ccc(OC(F)(F)F)cc1 |t:1| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50364776

(CHEMBL1952141 | CHEMBL1952329)Show SMILES CN1CCC(CNc2ccc3ncc(-c4cccc(OC(F)(F)F)c4)n3n2)CC1 Show InChI InChI=1S/C20H22F3N5O/c1-27-9-7-14(8-10-27)12-24-18-5-6-19-25-13-17(28(19)26-18)15-3-2-4-16(11-15)29-20(21,22)23/h2-6,11,13-14H,7-10,12H2,1H3,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) by coupled kinetic assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115724
BindingDB Entry DOI: 10.7270/Q2PC3607 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080288

(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-CGS-21,680 to human A2a receptor (hA2a) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080288

(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-CGS-21,680 to human A2a receptor (hA2a) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data