

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor 3 (RAT) | BDBM50166288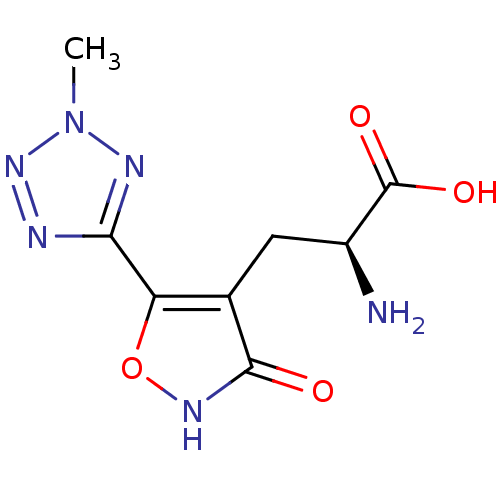 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.00220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Rattus norvegicus) | BDBM50166288 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR4 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50166288 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR2 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50166288 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR1 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561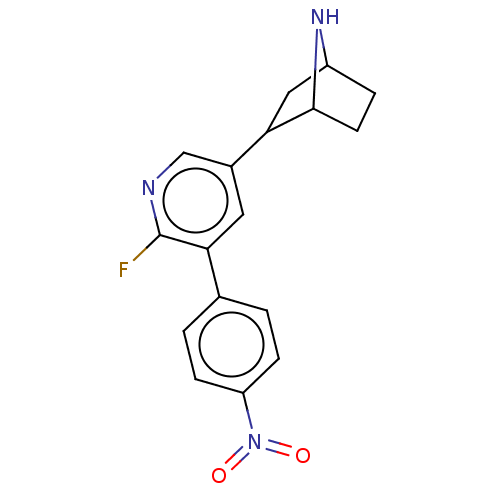 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM85357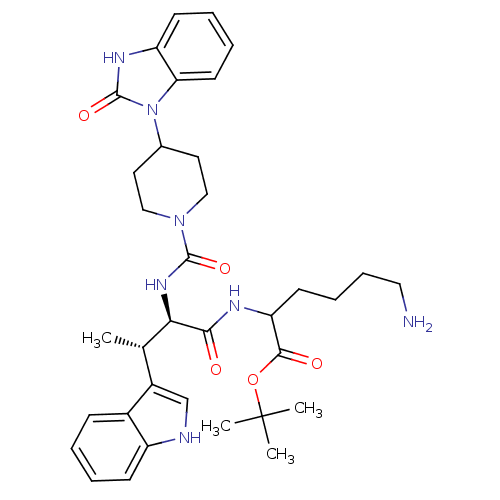 (2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Tested for binding affinity against human Coagulation factor Xa (trypsin-like serine protease) | Bioorg Med Chem Lett 12: 1651-5 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined | Bioorg Med Chem Lett 12: 1511-5 (2002) BindingDB Entry DOI: 10.7270/Q2P84B6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50002370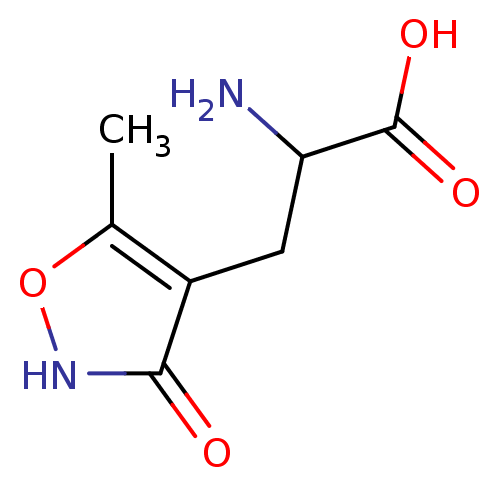 ((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR2 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50002370 ((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50002370 ((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR1 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50127165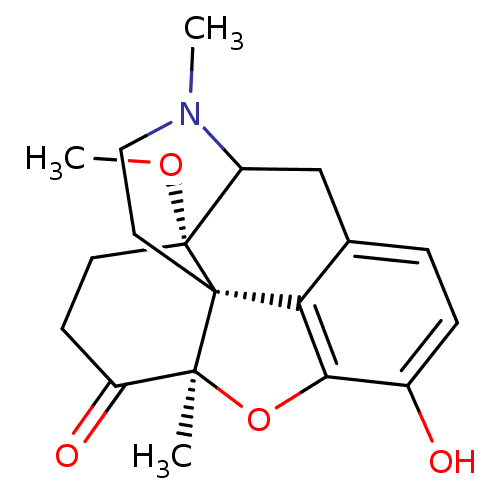 (10-hydroxy-17-methoxy-4,13-dimethyl-(13R,17S)-12-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description In vitro binding affinity at opioid receptor mu 1 was determined in C6 rat glioma cells using [3H]DAMGO | J Med Chem 46: 1758-63 (2003) Article DOI: 10.1021/jm021118o BindingDB Entry DOI: 10.7270/Q2V125JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM50395218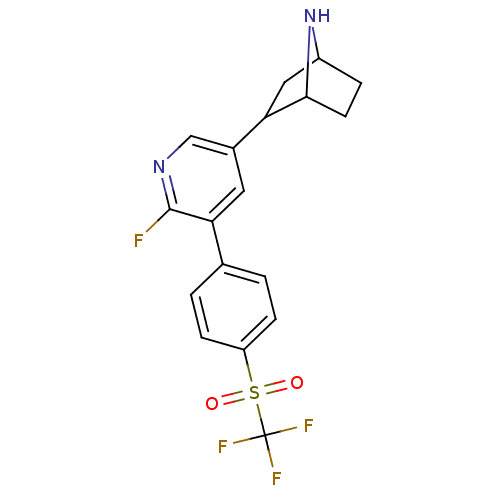 (CHEMBL2164666 | US9150581, RTI-7527-192) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198754 (CHEMBL3924888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Rattus norvegicus) | BDBM50002370 ((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR4 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM81767 (15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]p-CI-DPDPE in C6 glioma cells expressing the cloned Opioid receptor delta 1 | Bioorg Med Chem Lett 10: 2449-51 (2001) BindingDB Entry DOI: 10.7270/Q20K2939 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253328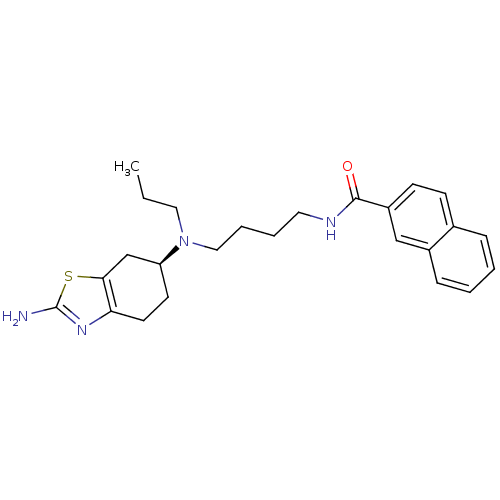 ((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50193861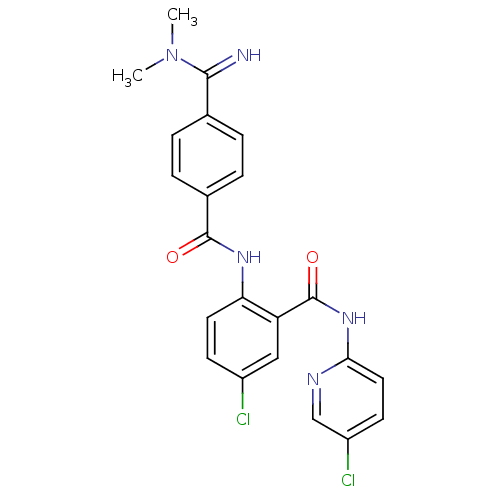 (5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Rattus norvegicus) | BDBM50166286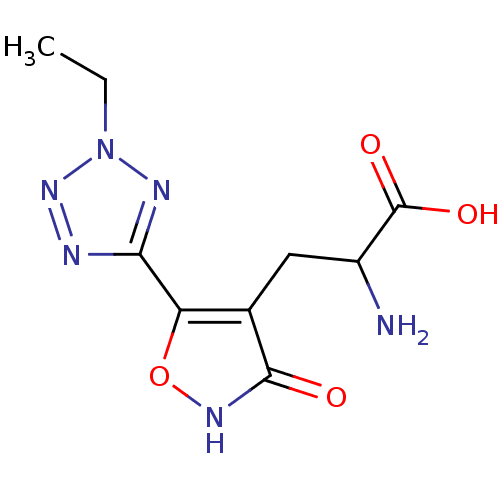 ((RS)-2-amino-3-[3-hydroxy-5-(2-ethyl-2H-5-tetrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR4 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50166286 ((RS)-2-amino-3-[3-hydroxy-5-(2-ethyl-2H-5-tetrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative farnesyl pyrophosphate synthase (Cryptosporidium parvum) | BDBM12578 (2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0500 | -61.2 | 30.1 | n/a | n/a | n/a | n/a | 7.7 | 37 |
University of Toronto | Assay Description Enzymatic assay using CpNPPPS was assayed using Reed and Rilling method with some modification. | Chem Biol 15: 1296-306 (2008) Article DOI: 10.1016/j.chembiol.2008.10.017 BindingDB Entry DOI: 10.7270/Q25B00XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM81766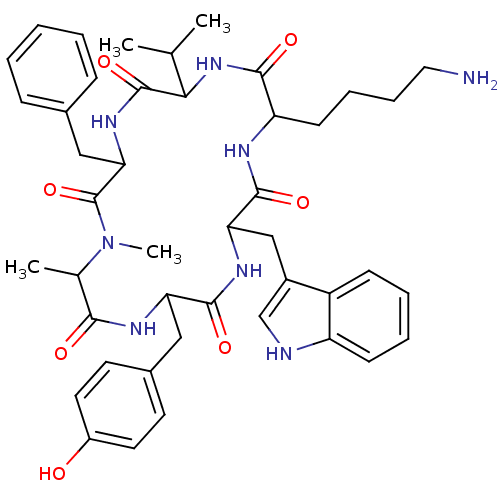 (CAS_3086456 | MK 678 | NSC_3086456) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463689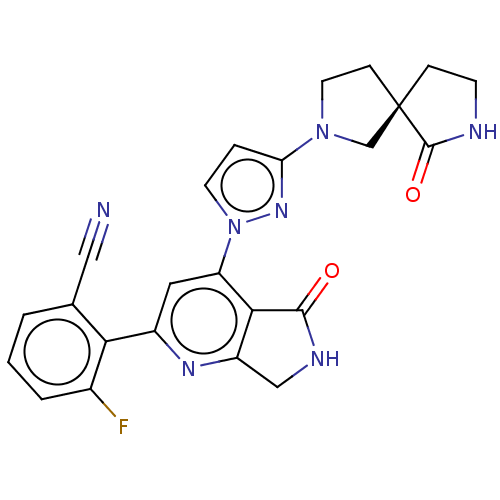 (US10781204, Compound I-166 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463600 (US10781204, Compound I-142 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463672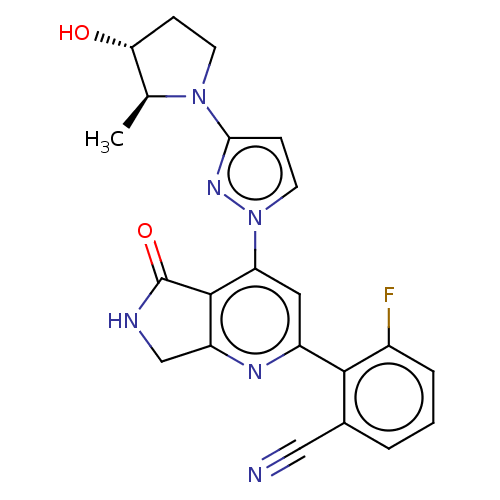 (US10781204, Compound I-150 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463682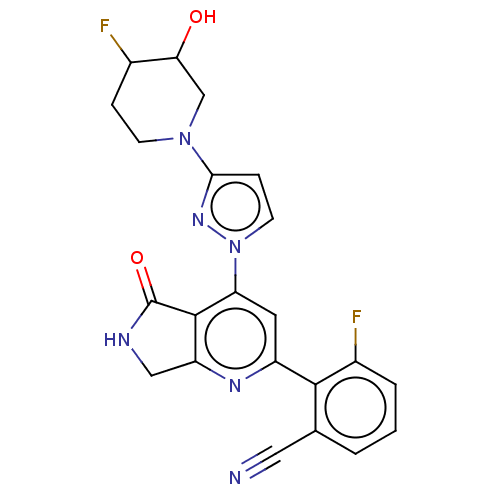 (US10781204, Compound I-160 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463621 (US10781204, Compound I-100 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463639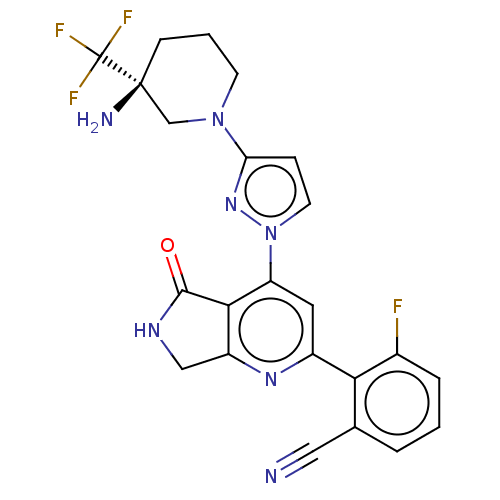 (US10781204, Compound I-118 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463640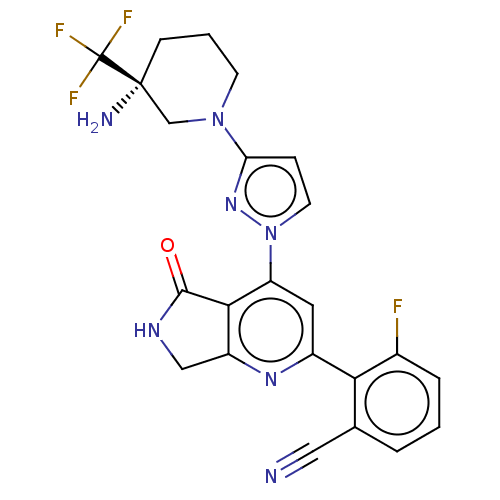 (US10781204, Compound I-119 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463591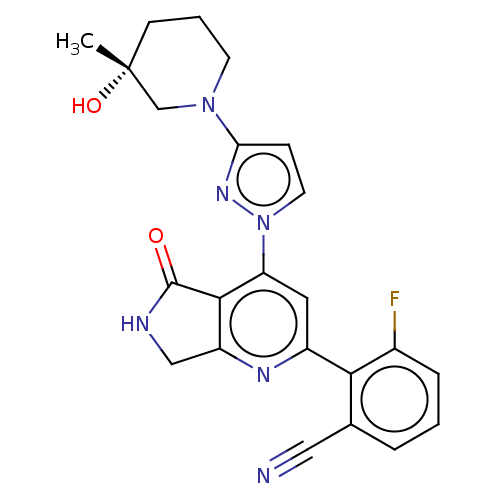 (US10781204, Compound I-111 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463596 (US10781204, Compound I-194 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463605 (US10781204, Compound I-84 | US11434240, Compound I...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463609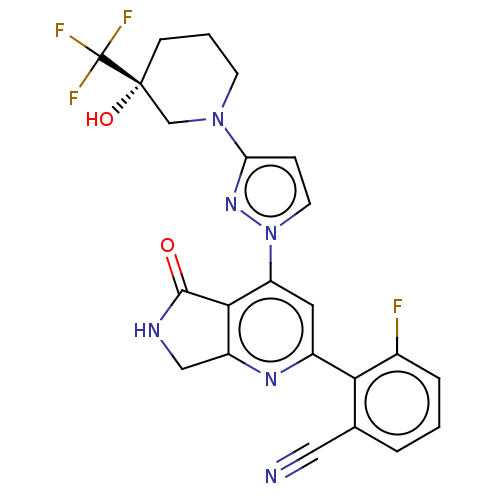 (US10781204, Compound I-88 | US11434240, Compound I...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463582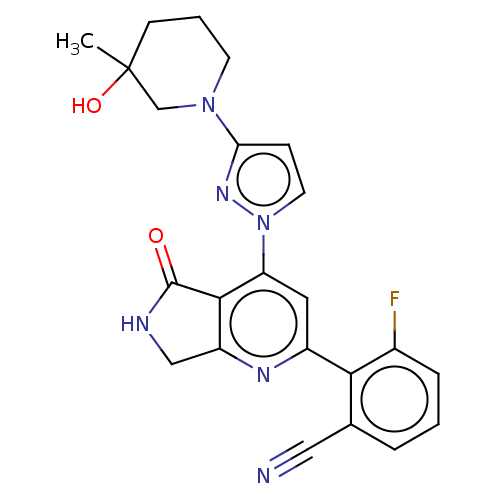 (US10781204, Compound I-186 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463736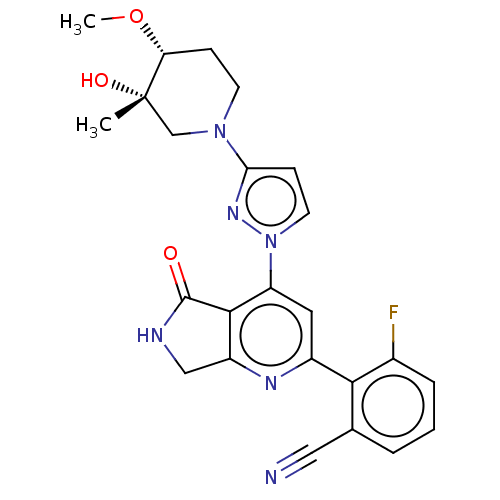 (US10781204, Compound I-213 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463752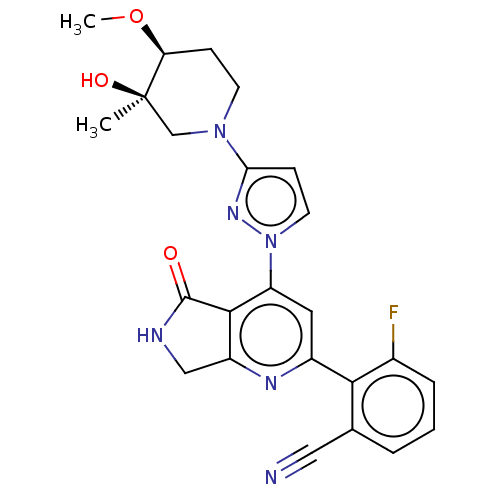 (US10781204, Compound I-229 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463710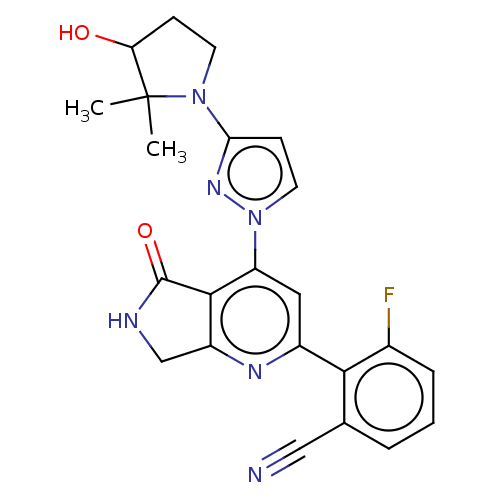 (US10781204, Compound I-187 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463711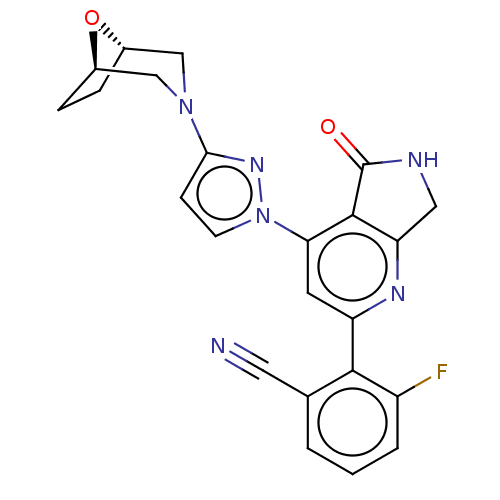 (US10781204, Compound I-188 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463671 (US10781204, Compound I-149 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463672 (US10781204, Compound I-150 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463682 (US10781204, Compound I-160 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463684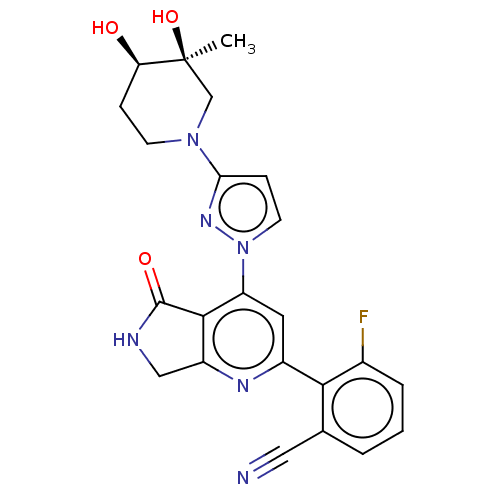 (US10781204, Compound I-162 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463671 (US10781204, Compound I-149 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463696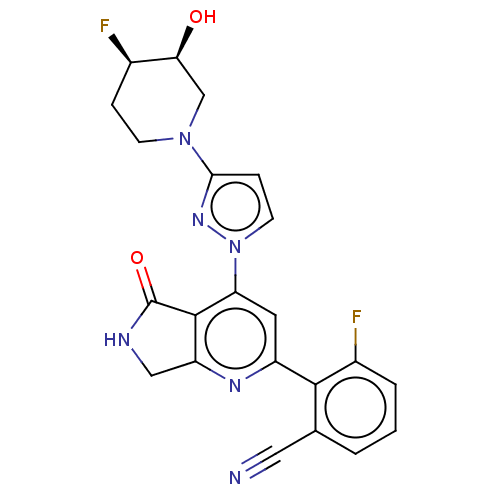 (US10781204, Compound I-173 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463639 (US10781204, Compound I-118 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463640 (US10781204, Compound I-119 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463657 (US10781204, Compound I-136 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 29752 total ) | Next | Last >> |