

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096105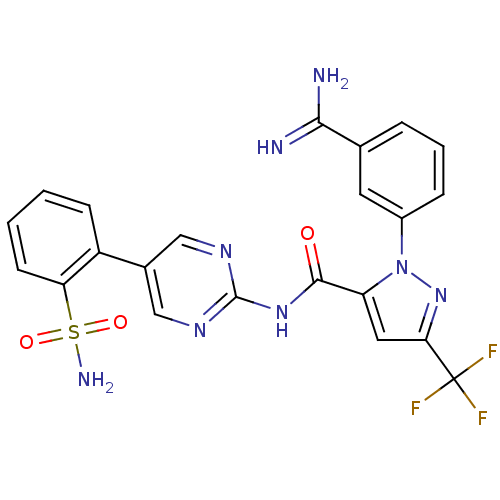 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096099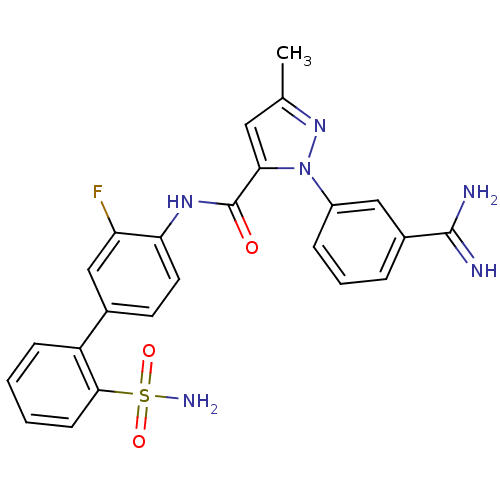 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096101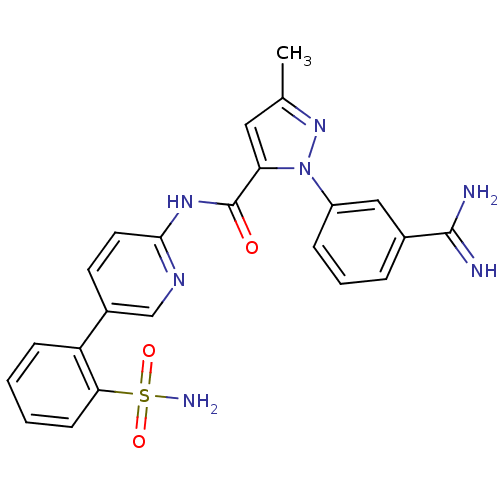 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096110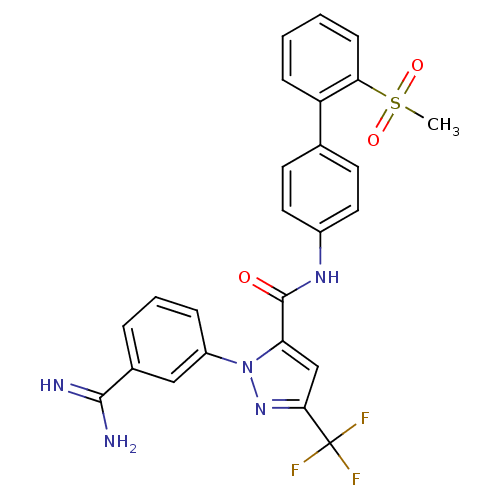 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096091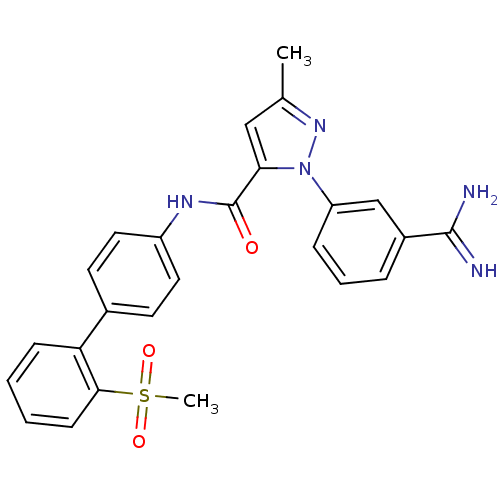 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096085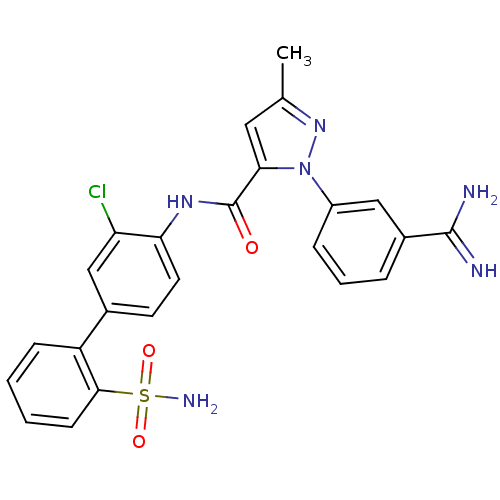 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096108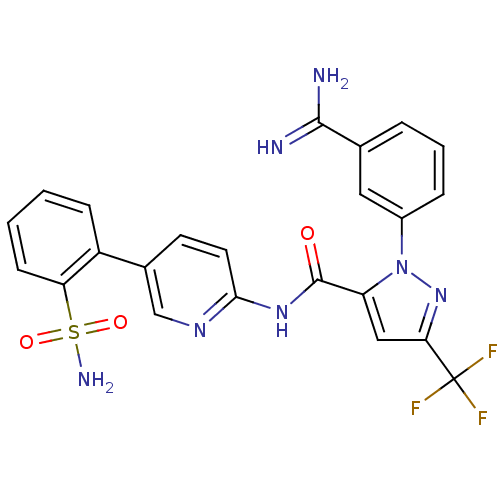 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096098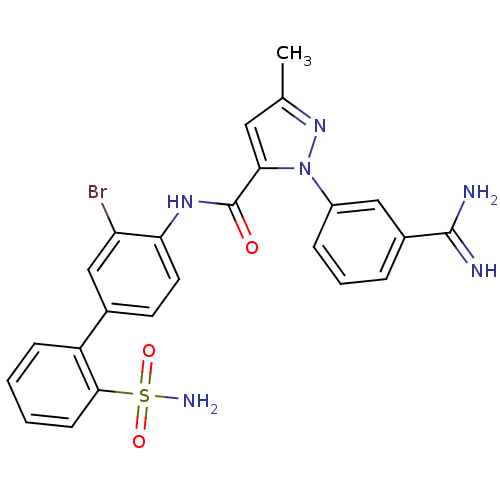 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648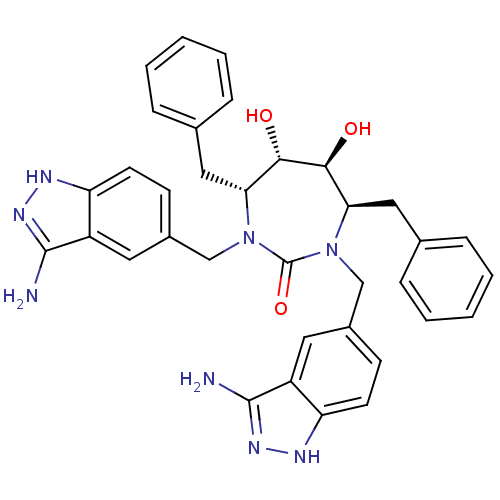 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36647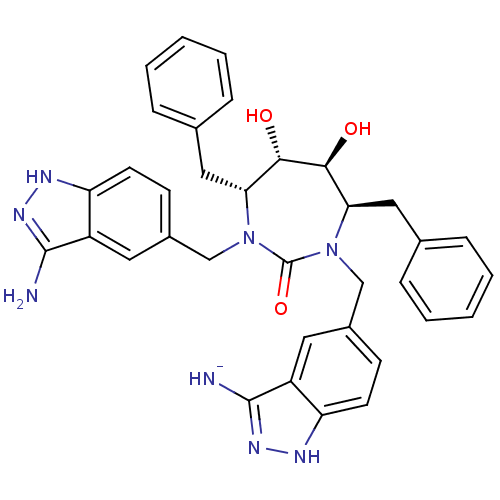 (3-Aminoindazole, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591155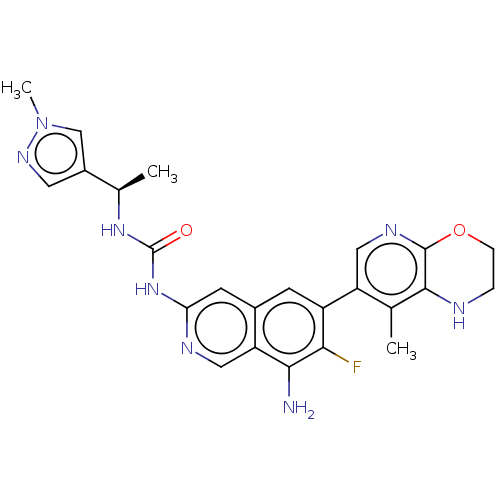 (US11566003, Compound 111) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591146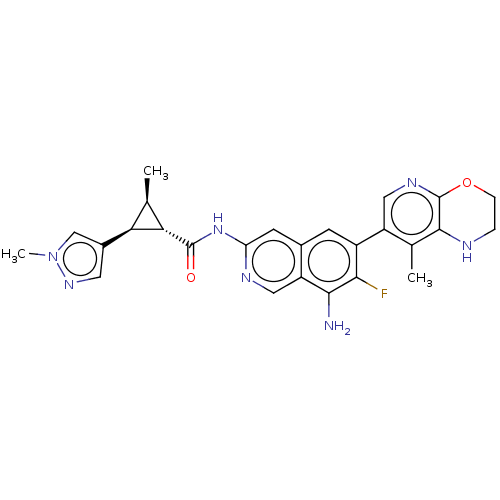 (US11566003, Compound 35) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591144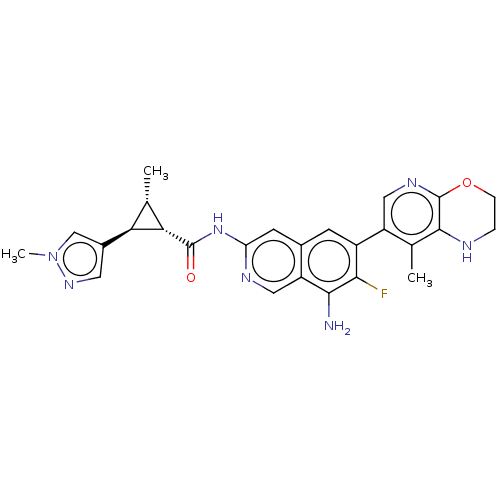 ((1R,2S,3R)-N-(8-amino-7-fluoro-6-(8-methyl-2,3-dih...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591252 (2-((8-amino-7-fluoro-6-(4-methyl-6- (oxazol-2-yl)p...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested in vitro for inhibition of human Coagulation factor X | J Med Chem 46: 5298-315 (2003) Article DOI: 10.1021/jm030212h BindingDB Entry DOI: 10.7270/Q2ZW1MP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591250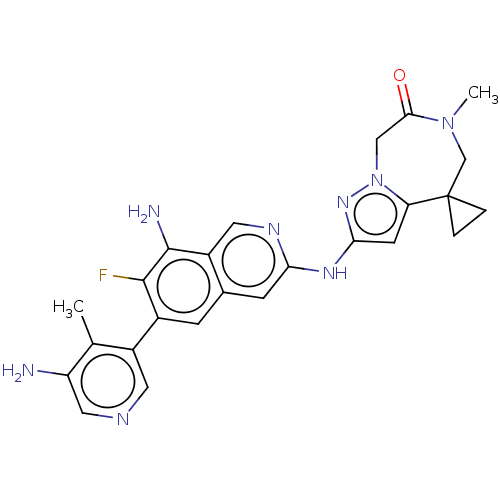 (2'-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-f...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591249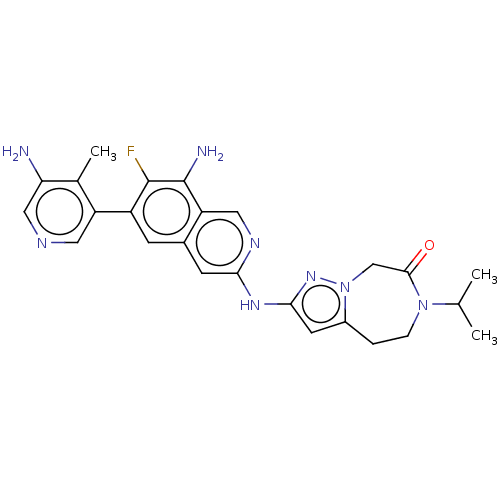 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591244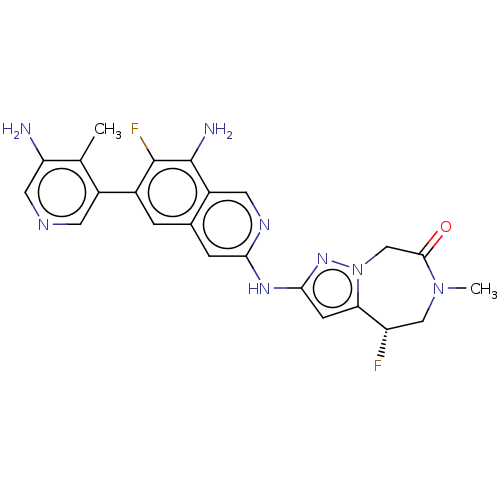 ((S)-2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591242 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591240 (2-((8-amino-6-(5-amino-4- chloropyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591137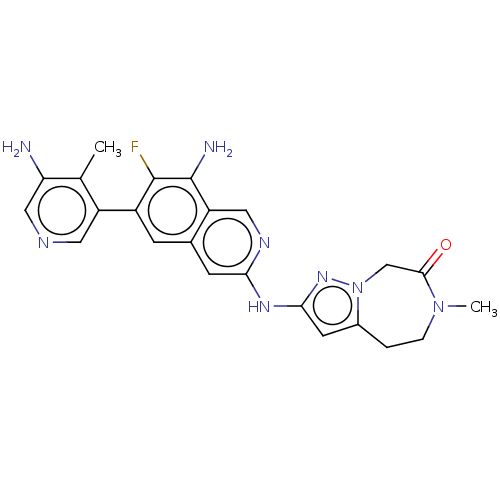 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7- f...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591137 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7- f...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. The solut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591211 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591217 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591220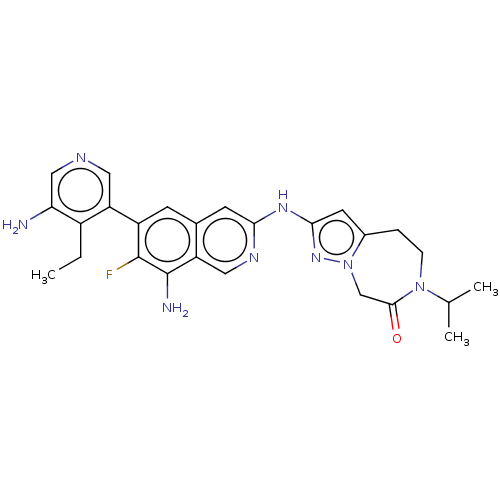 (2-((8-amino-6-(5-amino-4-ethylpyridin- 3-yl)-7-flu...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591222 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591225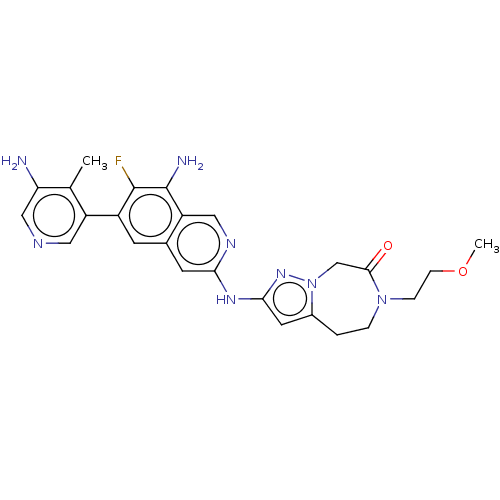 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | -61.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were determined ... | J Med Chem 46: 4405-18 (2003) Article DOI: 10.1021/jm020578e BindingDB Entry DOI: 10.7270/Q2TT4P78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591143 (1-(8-amino-7-fluoro-6-(8-methyl- 2,3-dihydro-1H-py...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM160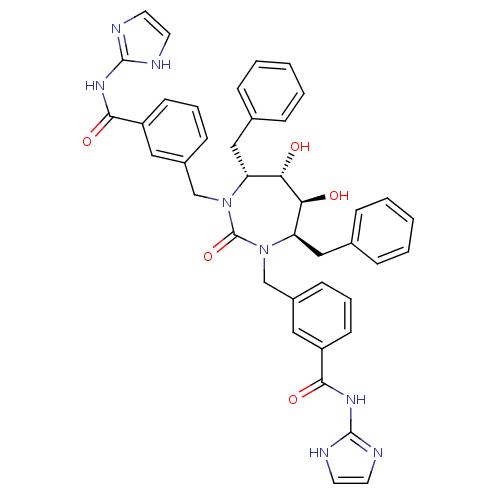 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591147 (US11566003, Compound 36) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591246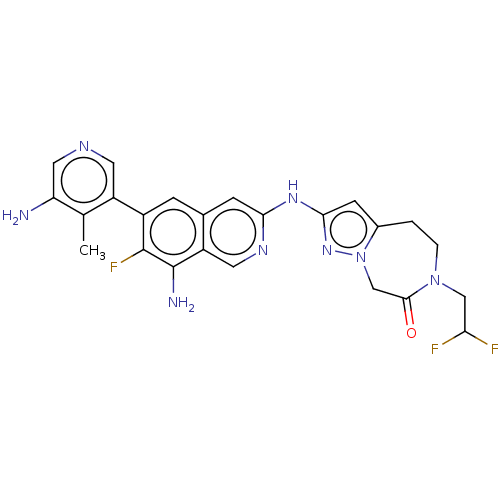 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591136 (2'-((8-amino-7-fluoro-6-(4- methylpyridin-3-yl)iso...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. The solut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096111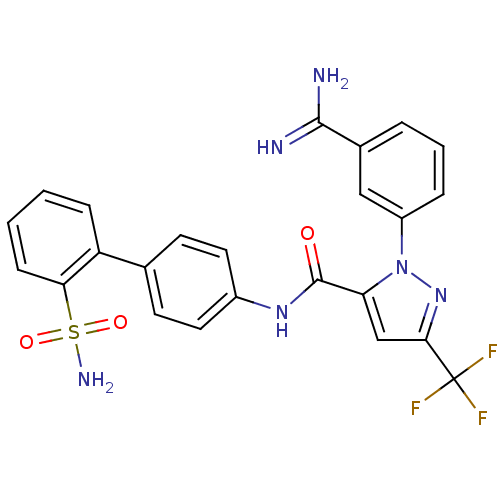 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591136 (2'-((8-amino-7-fluoro-6-(4- methylpyridin-3-yl)iso...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591177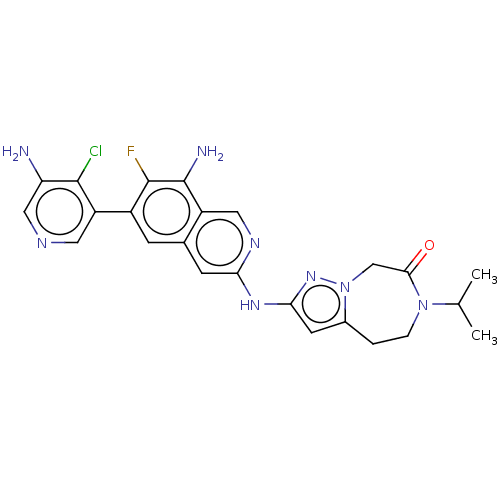 (2-((8-amino-6-(5-amino-4- chloropyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591139 (US11566003, Compound 9) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591141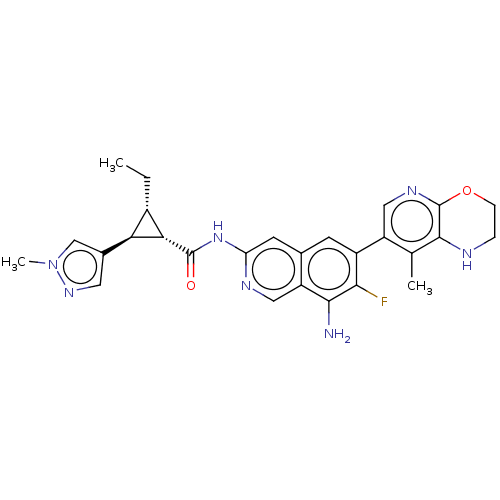 (US11566003, Compound 11) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065080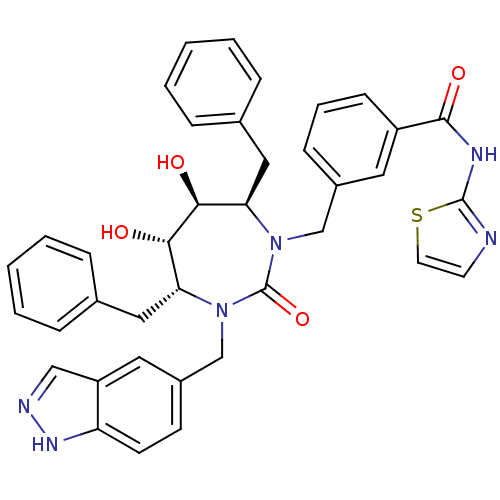 (3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065075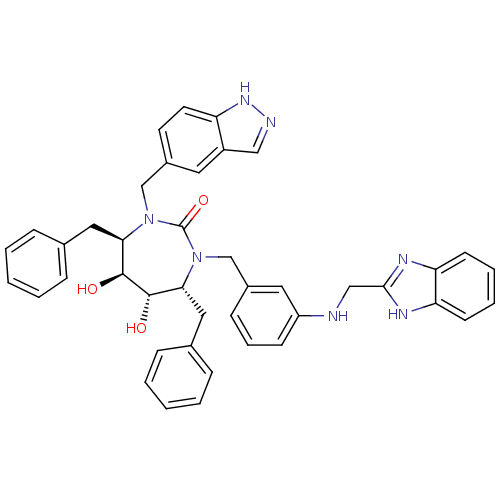 ((4R,5S,6S,7R)-1-{3-[(1H-Benzoimidazol-2-ylmethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36656 (Cyclobutylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591243 ((R)-2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591232 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591233 (2-((8-amino-6-(5-amino-4,6- dimethylpyridin-3-yl)-...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065089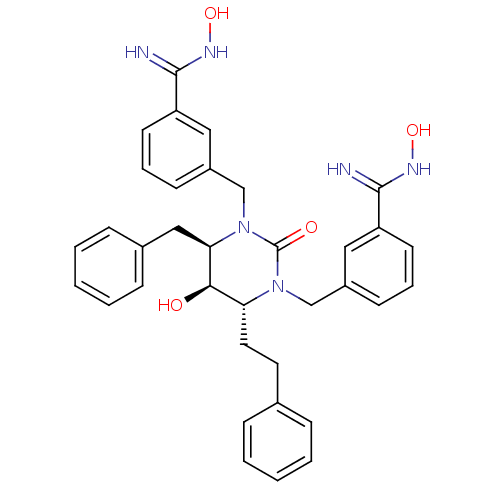 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamide oxime)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065078 ((4R,5S,6S,7R)-1-(3-Amino-4-fluoro-benzyl)-4,7-dibe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36649 (3-alkylaminoindazole cyclic urea, (Me)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591167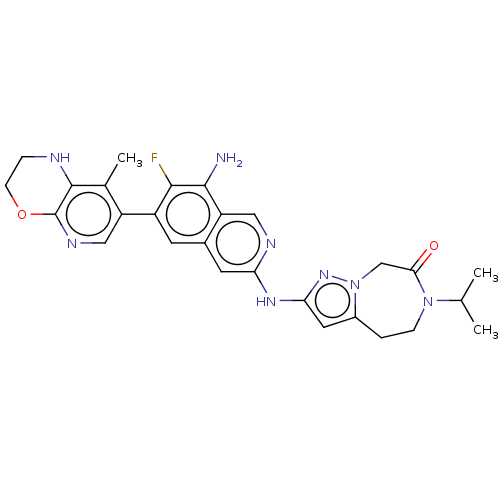 (2-((8-amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-p...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM161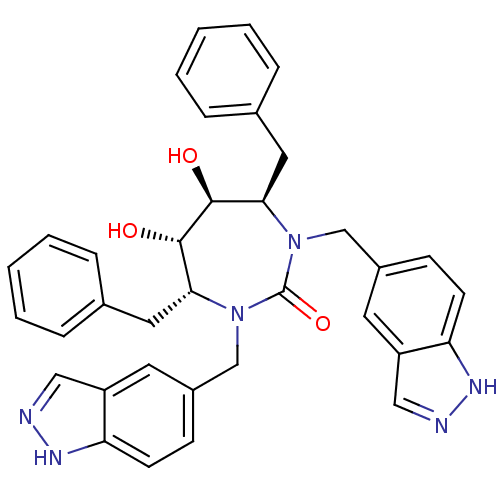 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4199 total ) | Next | Last >> |