 Found 4308 hits with Last Name = 'xi' and Initial = 's'
Found 4308 hits with Last Name = 'xi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50245852
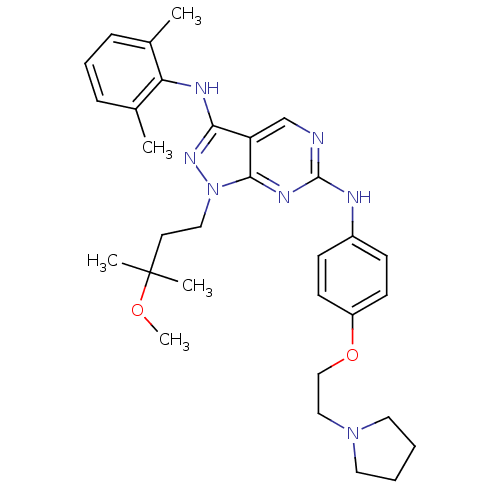
(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967
(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Binding affinity to EZH2 (unknown origin) |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50428107
(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50550643
(CHEMBL4749207)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(O)cc1)B(O)O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 20S constitutive proteasome beta 5 subunit assessed as equilibrium constant using fluorogenic peptide Ac-WLA-AMC as substra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01161
BindingDB Entry DOI: 10.7270/Q2K077W3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428109
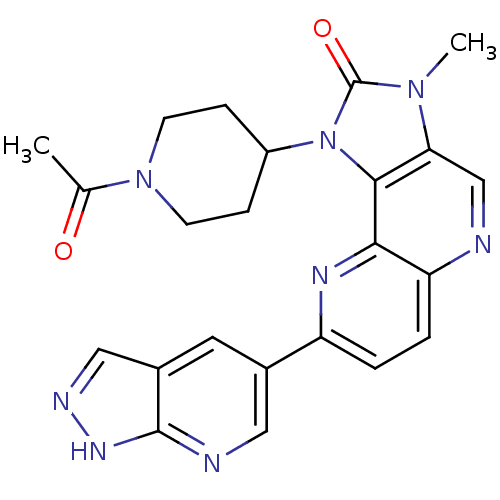
(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421256
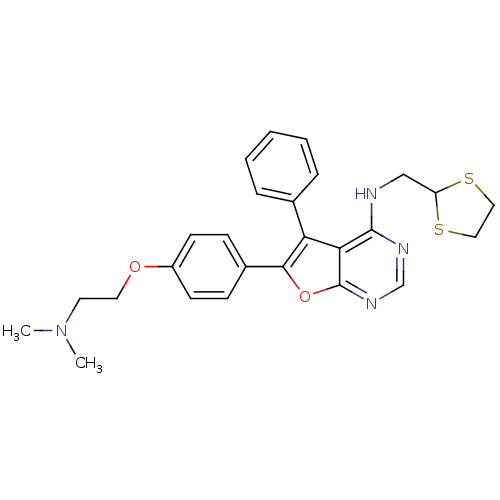
(CHEMBL2087874)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H28N4O2S2/c1-30(2)12-13-31-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)32-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50245852

(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421255
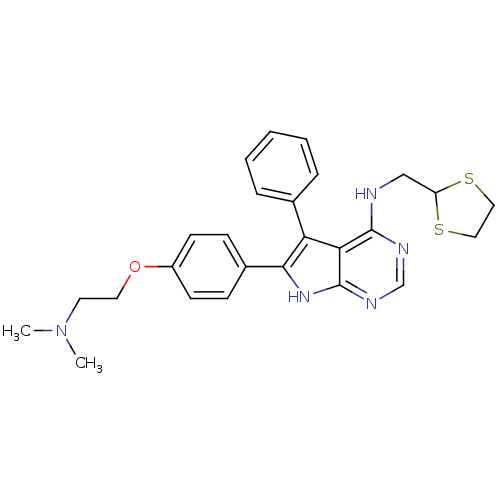
(CHEMBL2087873)Show SMILES CN(C)CCOc1ccc(cc1)-c1[nH]c2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H29N5OS2/c1-31(2)12-13-32-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)30-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421257
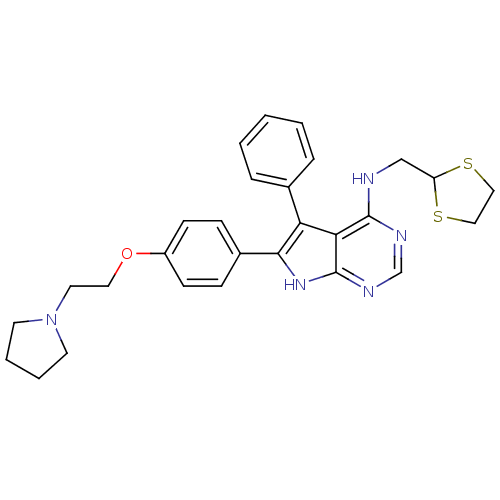
(CHEMBL2087875)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1[nH]c2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C28H31N5OS2/c1-2-6-20(7-3-1)24-25-27(29-18-23-35-16-17-36-23)30-19-31-28(25)32-26(24)21-8-10-22(11-9-21)34-15-14-33-12-4-5-13-33/h1-3,6-11,19,23H,4-5,12-18H2,(H2,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380320
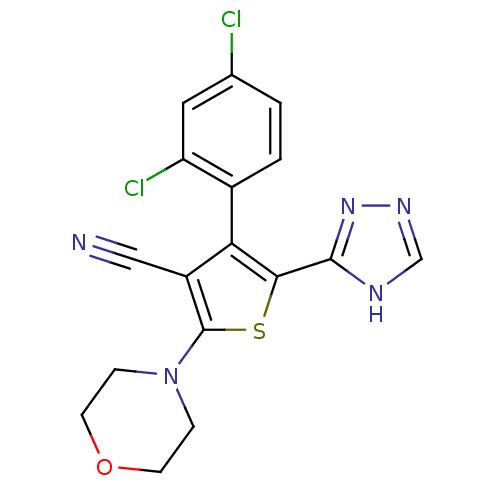
(CHEMBL2017653)Show SMILES Clc1ccc(-c2c(sc(N3CCOCC3)c2C#N)-c2nnc[nH]2)c(Cl)c1 |(13.97,-25.89,;13.2,-24.55,;13.97,-23.21,;13.2,-21.89,;11.67,-21.88,;10.91,-20.55,;11.39,-19.08,;10.14,-18.17,;8.88,-19.08,;7.54,-18.32,;7.53,-16.78,;6.2,-16.02,;4.87,-16.79,;4.88,-18.33,;6.21,-19.1,;9.36,-20.55,;8.46,-21.8,;7.56,-23.05,;12.84,-18.6,;14.09,-19.51,;15.33,-18.61,;14.86,-17.14,;13.32,-17.14,;10.89,-23.21,;9.36,-23.21,;11.66,-24.55,)| Show InChI InChI=1S/C17H13Cl2N5OS/c18-10-1-2-11(13(19)7-10)14-12(8-20)17(24-3-5-25-6-4-24)26-15(14)16-21-9-22-23-16/h1-2,7,9H,3-6H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428111
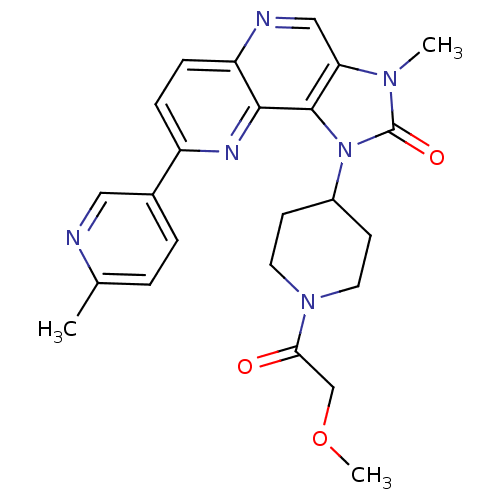
(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428108
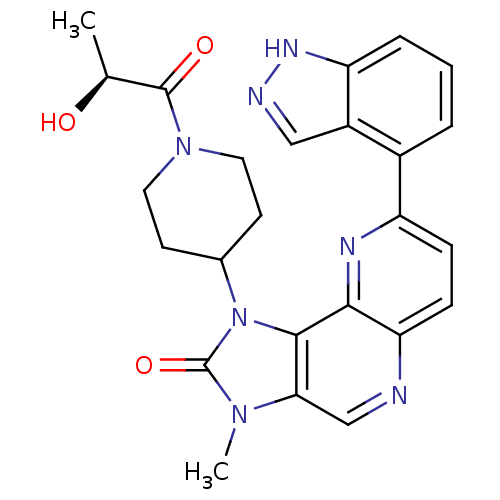
(CHEMBL2331669 | US8791131, 255)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O |r| Show InChI InChI=1S/C25H25N7O3/c1-14(33)24(34)31-10-8-15(9-11-31)32-23-21(30(2)25(32)35)13-26-20-7-6-18(28-22(20)23)16-4-3-5-19-17(16)12-27-29-19/h3-7,12-15,33H,8-11H2,1-2H3,(H,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428113
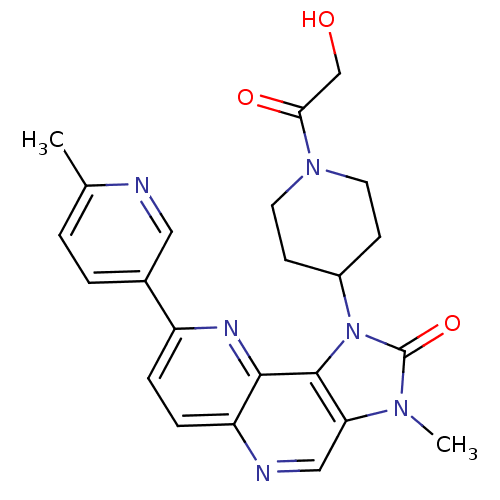
(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.532 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428115
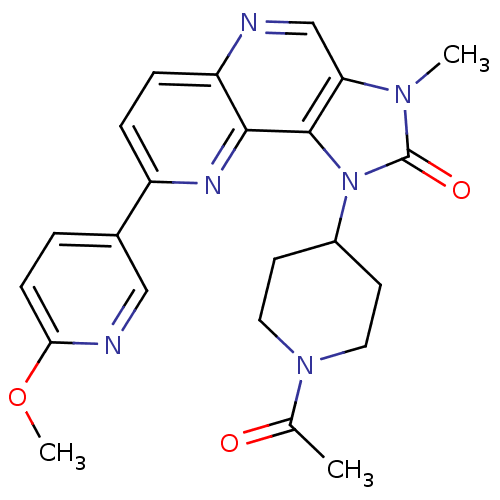
(CHEMBL2331661 | US8791131, 136)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(C)=O)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14(30)28-10-8-16(9-11-28)29-22-19(27(2)23(29)31)13-24-18-6-5-17(26-21(18)22)15-4-7-20(32-3)25-12-15/h4-7,12-13,16H,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380313
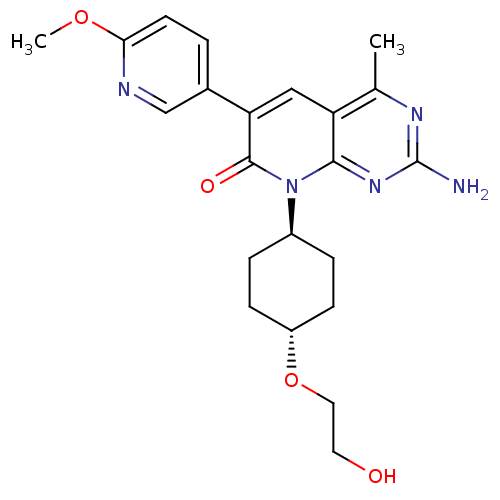
(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428110
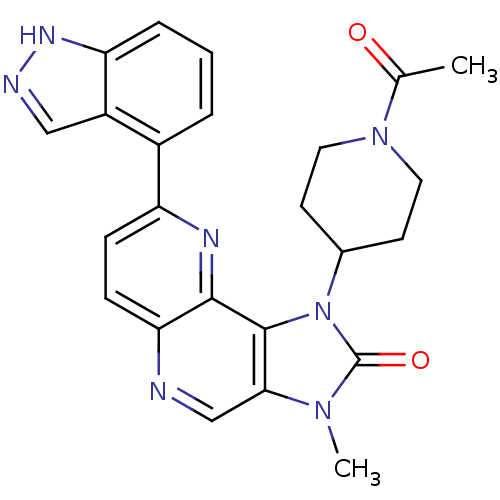
(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.584 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380321

(CHEMBL2017654)Show SMILES Fc1cc(ccc1-c1c(sc(N2CCOCC2)c1C#N)-c1nnc[nH]1)C#N |(23.39,-23.59,;24.93,-23.6,;25.69,-24.93,;27.23,-24.94,;28,-23.6,;27.24,-22.27,;25.7,-22.27,;24.94,-20.94,;25.42,-19.47,;24.17,-18.56,;22.91,-19.47,;21.57,-18.7,;21.56,-17.16,;20.23,-16.4,;18.89,-17.18,;18.9,-18.72,;20.24,-19.48,;23.39,-20.94,;22.49,-22.19,;21.58,-23.43,;26.88,-18.99,;28.12,-19.89,;29.37,-18.99,;28.89,-17.52,;27.35,-17.52,;28,-26.27,;28.77,-27.6,)| Show InChI InChI=1S/C18H13FN6OS/c19-14-7-11(8-20)1-2-12(14)15-13(9-21)18(25-3-5-26-6-4-25)27-16(15)17-22-10-23-24-17/h1-2,7,10H,3-6H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361649
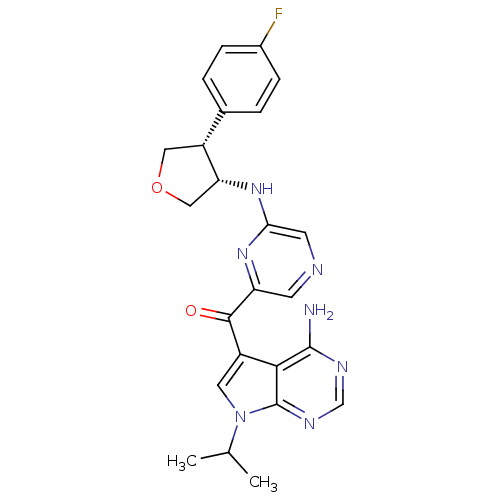
(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361648
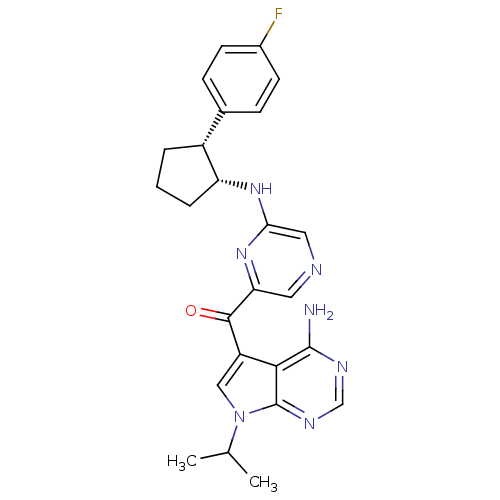
(CHEMBL1940246)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H26FN7O/c1-14(2)33-12-18(22-24(27)29-13-30-25(22)33)23(34)20-10-28-11-21(32-20)31-19-5-3-4-17(19)15-6-8-16(26)9-7-15/h6-14,17,19H,3-5H2,1-2H3,(H,31,32)(H2,27,29,30)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361642
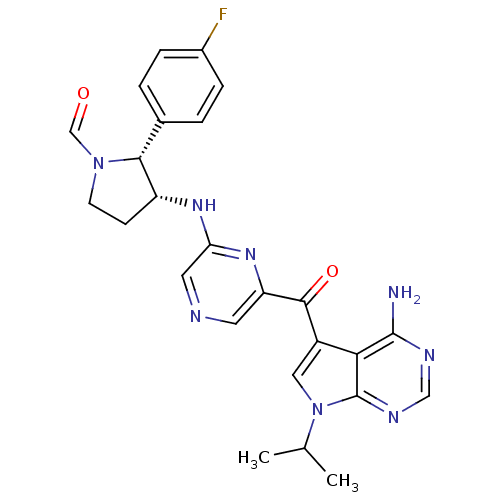
(CHEMBL1940251)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN(C=O)[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25FN8O2/c1-14(2)34-11-17(21-24(27)29-12-30-25(21)34)23(36)19-9-28-10-20(32-19)31-18-7-8-33(13-35)22(18)15-3-5-16(26)6-4-15/h3-6,9-14,18,22H,7-8H2,1-2H3,(H,31,32)(H2,27,29,30)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428113

(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361641
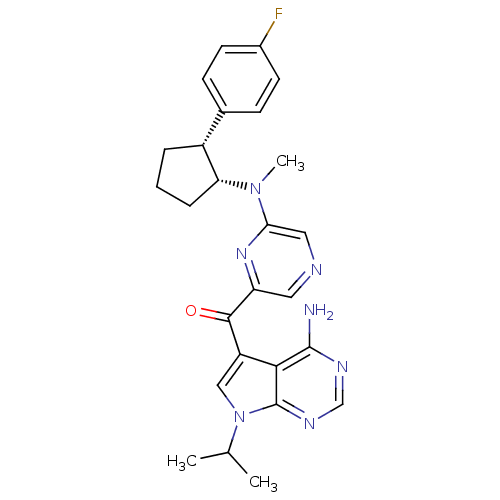
(CHEMBL1940247)Show SMILES CC(C)n1cc(C(=O)c2cncc(n2)N(C)[C@@H]2CCC[C@@H]2c2ccc(F)cc2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28FN7O/c1-15(2)34-13-19(23-25(28)30-14-31-26(23)34)24(35)20-11-29-12-22(32-20)33(3)21-6-4-5-18(21)16-7-9-17(27)10-8-16/h7-15,18,21H,4-6H2,1-3H3,(H2,28,30,31)/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428111

(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361652
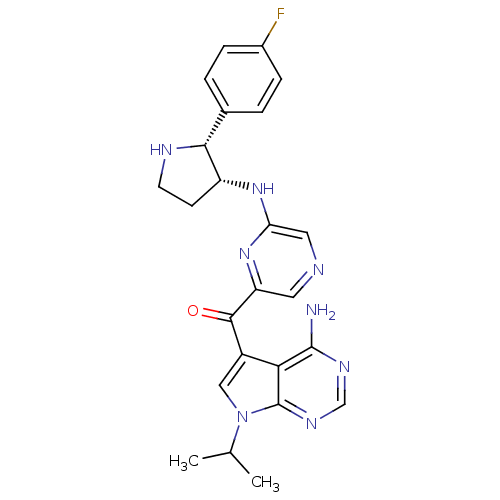
(CHEMBL1940250)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-16(20-23(26)29-12-30-24(20)33)22(34)18-9-27-10-19(32-18)31-17-7-8-28-21(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,21,28H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-2 (unknown origin) fluorescence polarization assay |
Bioorg Med Chem 21: 11-20 (2012)
Article DOI: 10.1016/j.bmc.2012.11.008
BindingDB Entry DOI: 10.7270/Q25X2B7H |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361650
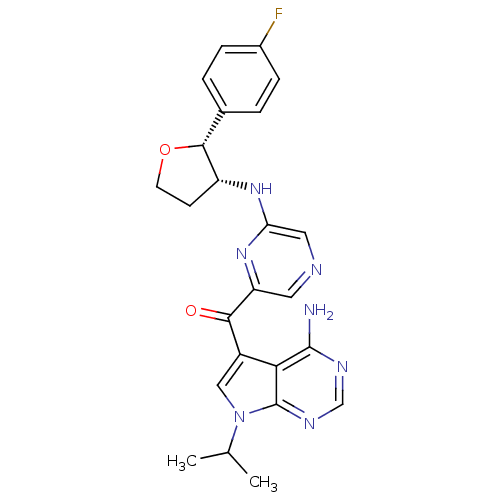
(CHEMBL1940248)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCO[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-11-16(20-23(26)28-12-29-24(20)32)21(33)18-9-27-10-19(31-18)30-17-7-8-34-22(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,22H,7-8H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361643
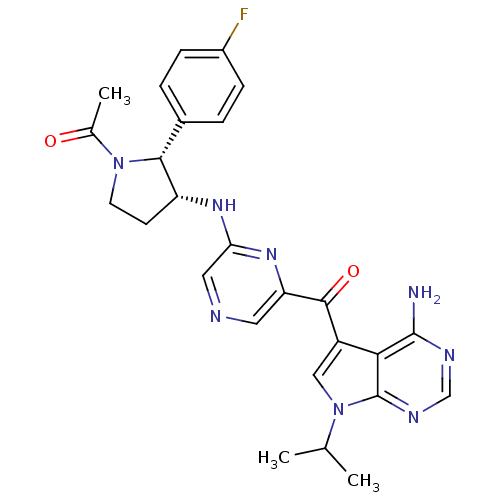
(CHEMBL1940252)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccc(F)cc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H27FN8O2/c1-14(2)35-12-18(22-25(28)30-13-31-26(22)35)24(37)20-10-29-11-21(33-20)32-19-8-9-34(15(3)36)23(19)16-4-6-17(27)7-5-16/h4-7,10-14,19,23H,8-9H2,1-3H3,(H,32,33)(H2,28,30,31)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361644
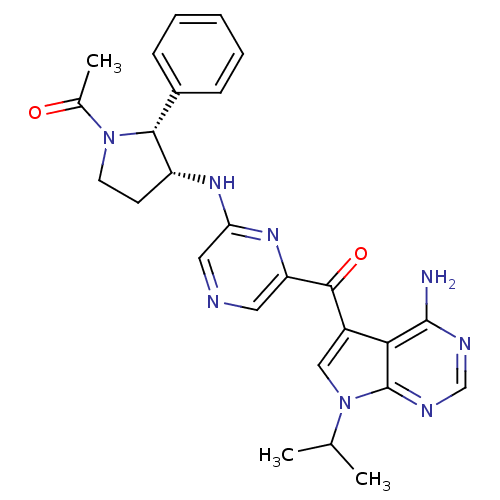
(CHEMBL1940253)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccccc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28N8O2/c1-15(2)34-13-18(22-25(27)29-14-30-26(22)34)24(36)20-11-28-12-21(32-20)31-19-9-10-33(16(3)35)23(19)17-7-5-4-6-8-17/h4-8,11-15,19,23H,9-10H2,1-3H3,(H,31,32)(H2,27,29,30)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428117
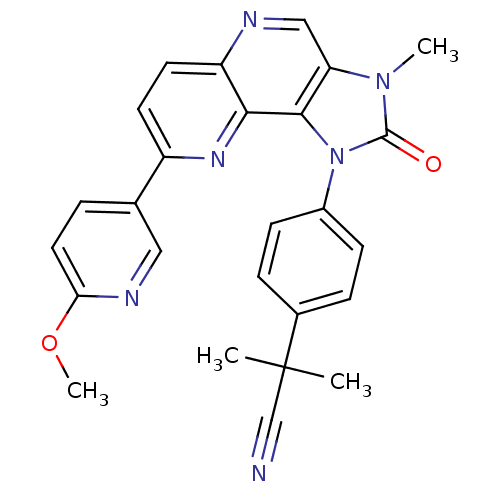
(CHEMBL2331657)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(cc4)C(C)(C)C#N)c3c2n1 Show InChI InChI=1S/C26H22N6O2/c1-26(2,15-27)17-6-8-18(9-7-17)32-24-21(31(3)25(32)33)14-28-20-11-10-19(30-23(20)24)16-5-12-22(34-4)29-13-16/h5-14H,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428110

(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM20800
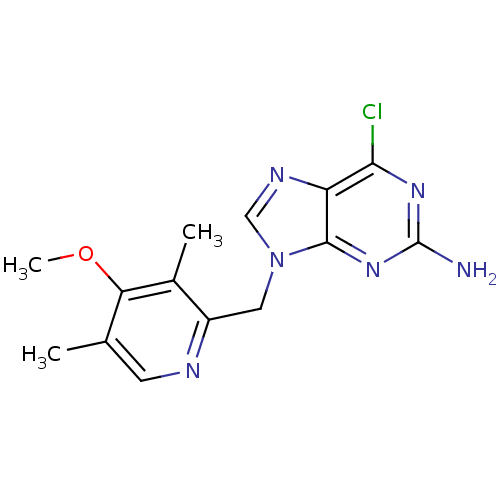
(2-amino-6-halopurine analogue, 20 | 6-chloro-9-[(4...)Show InChI InChI=1S/C14H15ClN6O/c1-7-4-17-9(8(2)11(7)22-3)5-21-6-18-10-12(15)19-14(16)20-13(10)21/h4,6H,5H2,1-3H3,(H2,16,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged full length human HSP90 (9 to 236 residues) expressed in Escherichia coli BL21(DE3) cells after 18 hrs by fluore... |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380314
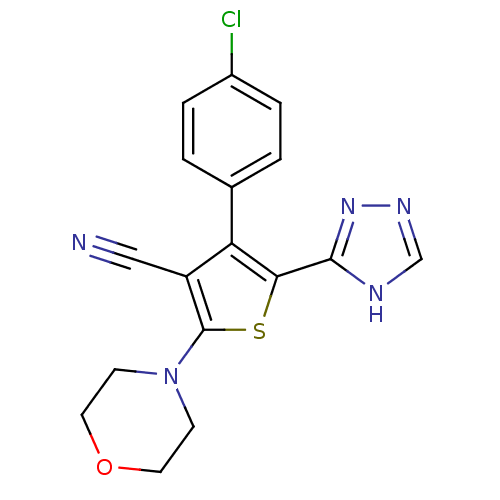
(CHEMBL2017648)Show SMILES Clc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1nnc[nH]1 Show InChI InChI=1S/C17H14ClN5OS/c18-12-3-1-11(2-4-12)14-13(9-19)17(23-5-7-24-8-6-23)25-15(14)16-20-10-21-22-16/h1-4,10H,5-8H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380315
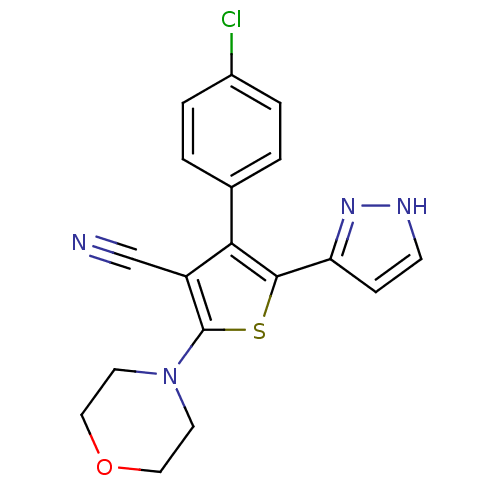
(CHEMBL2017649)Show SMILES Clc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1cc[nH]n1 Show InChI InChI=1S/C18H15ClN4OS/c19-13-3-1-12(2-4-13)16-14(11-20)18(23-7-9-24-10-8-23)25-17(16)15-5-6-21-22-15/h1-6H,7-10H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
ACS Med Chem Lett 2: 809-813 (2011)
Article DOI: 10.1021/ml200126j
BindingDB Entry DOI: 10.7270/Q2DJ5GN2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159347
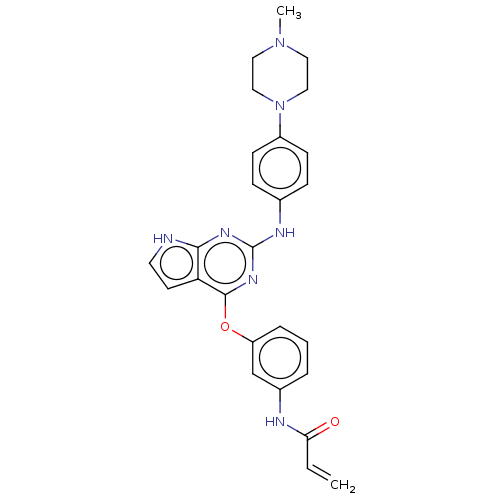
(CHEMBL3787662 | US9586965, Cpd 1)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C26H27N7O2/c1-3-23(34)28-19-5-4-6-21(17-19)35-25-22-11-12-27-24(22)30-26(31-25)29-18-7-9-20(10-8-18)33-15-13-32(2)14-16-33/h3-12,17H,1,13-16H2,2H3,(H,28,34)(H2,27,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50245854

(CHEMBL504331 | N3-(2,6-dichlorophenyl)-N6-(3-fluor...)Show SMILES COC(C)(C)CCn1nc(Nc2c(Cl)cccc2Cl)c2cnc(Nc3ccc(OCCN4CCCC4)c(F)c3)nc12 Show InChI InChI=1S/C29H34Cl2FN7O2/c1-29(2,40-3)11-14-39-27-20(26(37-39)35-25-21(30)7-6-8-22(25)31)18-33-28(36-27)34-19-9-10-24(23(32)17-19)41-16-15-38-12-4-5-13-38/h6-10,17-18H,4-5,11-16H2,1-3H3,(H,35,37)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428118
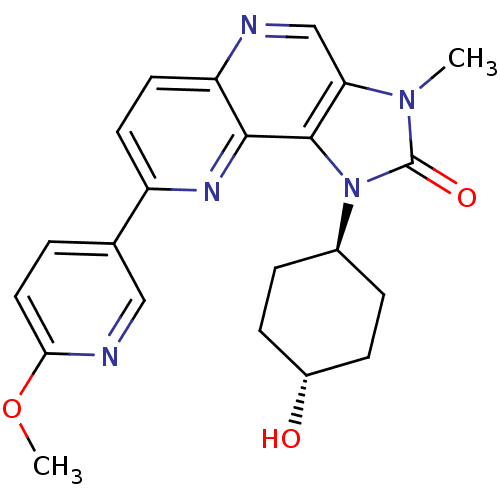
(CHEMBL2331659 | US8791131, 134)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@H](O)CC4)c3c2n1 |r,wU:20.20,wD:23.24,(32.03,-7.87,;33.36,-7.1,;34.69,-7.87,;34.69,-9.41,;36.03,-10.18,;37.36,-9.41,;37.36,-7.86,;36.02,-7.1,;38.7,-10.18,;38.69,-11.72,;40.02,-12.49,;41.35,-11.71,;42.69,-12.48,;44.03,-11.7,;44.01,-10.15,;45.14,-9.12,;46.65,-9.43,;44.51,-7.72,;45.28,-6.39,;42.99,-7.89,;42.18,-6.59,;40.64,-6.65,;39.83,-5.35,;40.55,-3.98,;39.73,-2.68,;42.09,-3.93,;42.9,-5.23,;42.68,-9.39,;41.35,-10.17,;40.02,-9.41,)| Show InChI InChI=1S/C22H23N5O3/c1-26-18-12-23-17-9-8-16(13-3-10-19(30-2)24-11-13)25-20(17)21(18)27(22(26)29)14-4-6-15(28)7-5-14/h3,8-12,14-15,28H,4-7H2,1-2H3/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421276
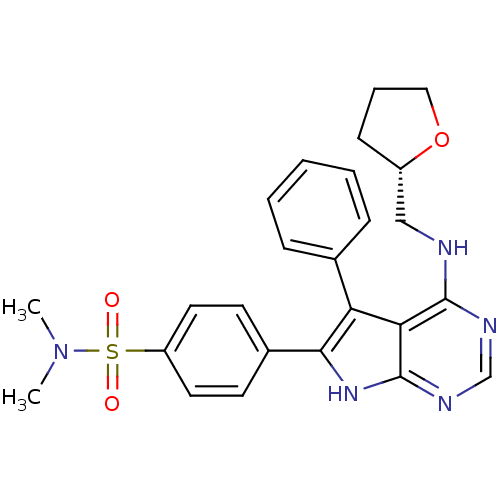
(CHEMBL2087652)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C25H27N5O3S/c1-30(2)34(31,32)20-12-10-18(11-13-20)23-21(17-7-4-3-5-8-17)22-24(27-16-28-25(22)29-23)26-15-19-9-6-14-33-19/h3-5,7-8,10-13,16,19H,6,9,14-15H2,1-2H3,(H2,26,27,28,29)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421278
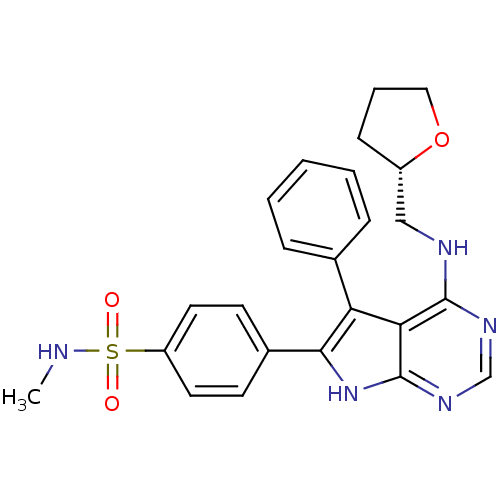
(CHEMBL2087654)Show SMILES CNS(=O)(=O)c1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C24H25N5O3S/c1-25-33(30,31)19-11-9-17(10-12-19)22-20(16-6-3-2-4-7-16)21-23(27-15-28-24(21)29-22)26-14-18-8-5-13-32-18/h2-4,6-7,9-12,15,18,25H,5,8,13-14H2,1H3,(H2,26,27,28,29)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159358
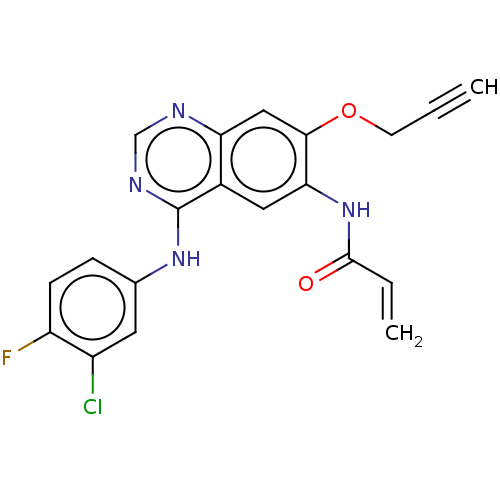
(CHEMBL3787386)Show SMILES Fc1ccc(Nc2ncnc3cc(OCC#C)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C20H14ClFN4O2/c1-3-7-28-18-10-16-13(9-17(18)26-19(27)4-2)20(24-11-23-16)25-12-5-6-15(22)14(21)8-12/h1,4-6,8-11H,2,7H2,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159358

(CHEMBL3787386)Show SMILES Fc1ccc(Nc2ncnc3cc(OCC#C)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C20H14ClFN4O2/c1-3-7-28-18-10-16-13(9-17(18)26-19(27)4-2)20(24-11-23-16)25-12-5-6-15(22)14(21)8-12/h1,4-6,8-11H,2,7H2,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50246214
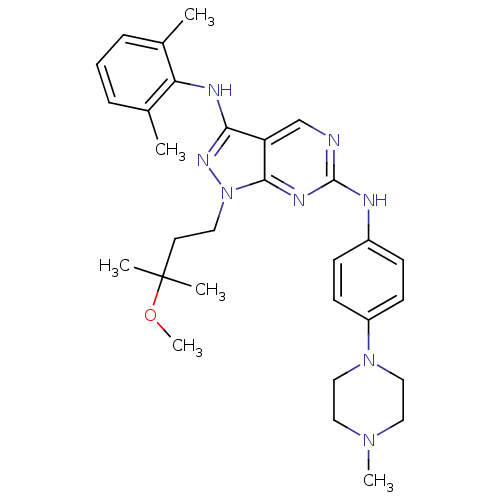
(CHEMBL487897 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(cc3)N3CCN(C)CC3)nc12 Show InChI InChI=1S/C30H40N8O/c1-21-8-7-9-22(2)26(21)33-27-25-20-31-29(34-28(25)38(35-27)15-14-30(3,4)39-6)32-23-10-12-24(13-11-23)37-18-16-36(5)17-19-37/h7-13,20H,14-19H2,1-6H3,(H,33,35)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50246162
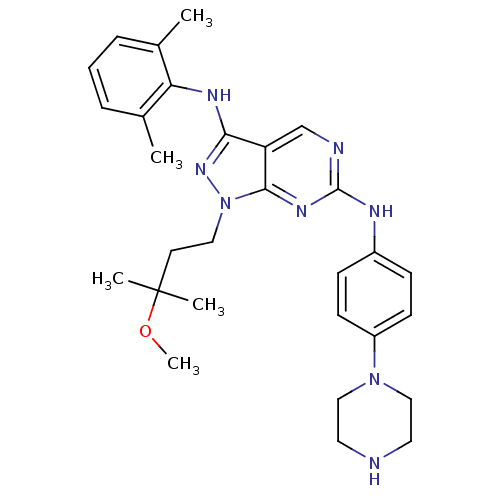
(CHEMBL472392 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(cc3)N3CCNCC3)nc12 Show InChI InChI=1S/C29H38N8O/c1-20-7-6-8-21(2)25(20)33-26-24-19-31-28(34-27(24)37(35-26)16-13-29(3,4)38-5)32-22-9-11-23(12-10-22)36-17-14-30-15-18-36/h6-12,19,30H,13-18H2,1-5H3,(H,33,35)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361653
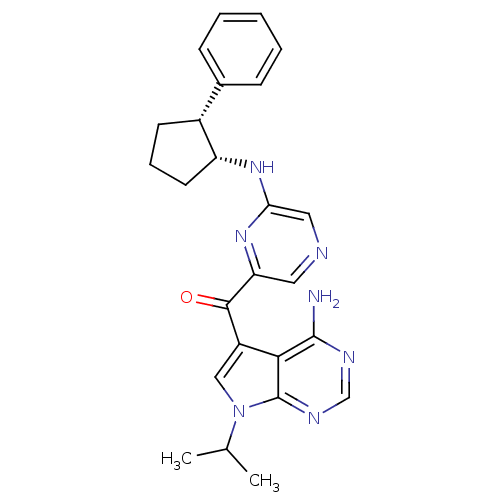
(CHEMBL1940245)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccccc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H27N7O/c1-15(2)32-13-18(22-24(26)28-14-29-25(22)32)23(33)20-11-27-12-21(31-20)30-19-10-6-9-17(19)16-7-4-3-5-8-16/h3-5,7-8,11-15,17,19H,6,9-10H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data