

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine esterase (Homo sapiens (Human)) | BDBM50552232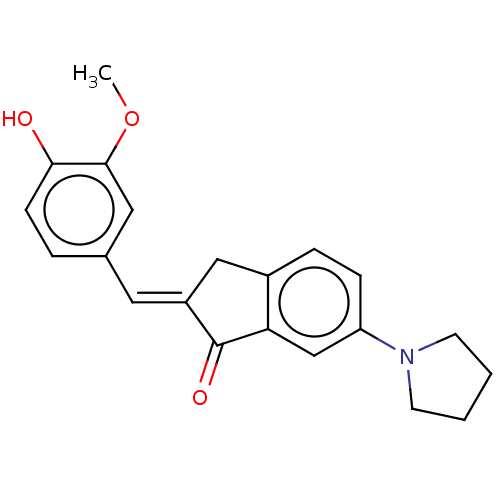 (CHEMBL4776624) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of hCES2A in human liver microsome assessed as reduction in fluorescein diacetate hydrolysis preincubated for 10 mins followed by su... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112856 BindingDB Entry DOI: 10.7270/Q2NZ8CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50017698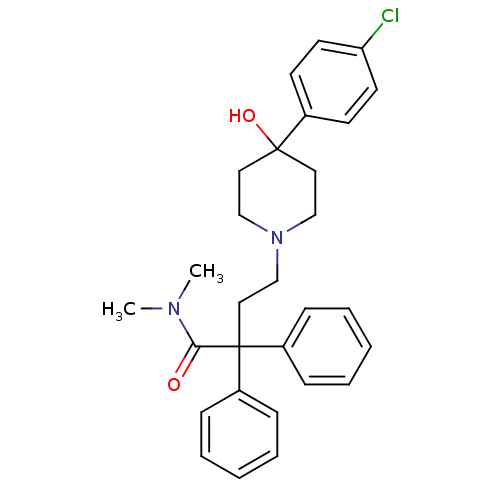 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CES2A using 4-methylumbelliferone as a substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112856 BindingDB Entry DOI: 10.7270/Q2NZ8CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312702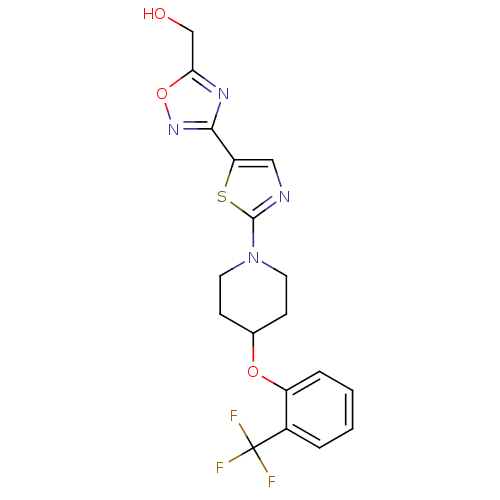 ((3-(2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312702 ((3-(2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50049041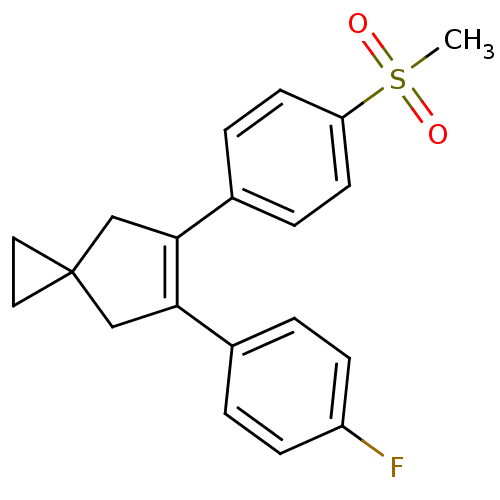 (5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288289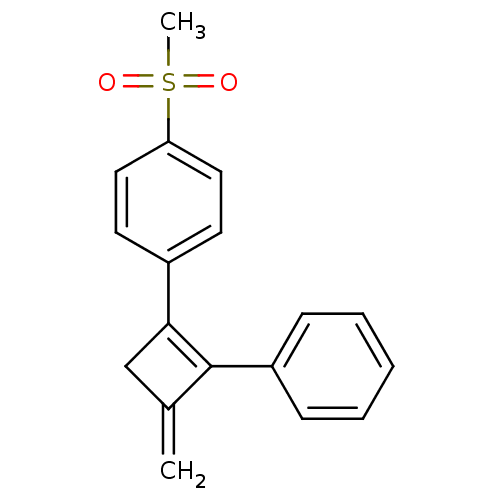 (1-Methanesulfonyl-4-(3-methylene-2-phenyl-cyclobut...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600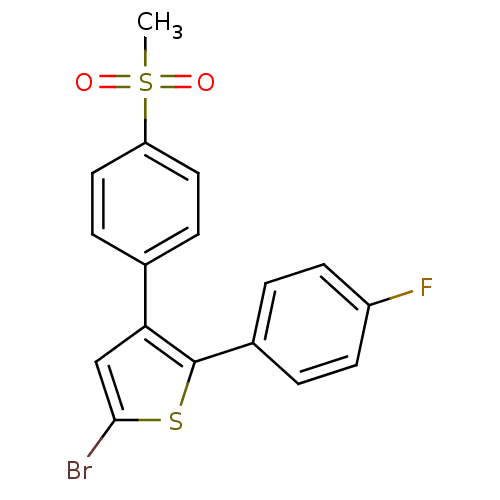 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of PGE-2 produced by arachidonic acid-stimulated CHO cells stably expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 8: 2777-82 (1999) BindingDB Entry DOI: 10.7270/Q2FX78M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288292 (1-(4,4-Dimethyl-3-methylene-2-phenyl-cyclobut-1-en...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288291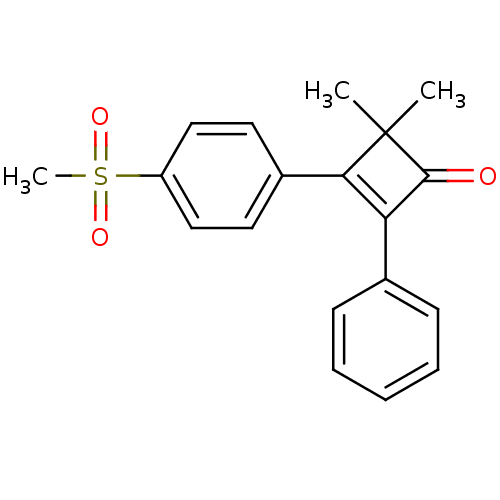 (3-(4-Methanesulfonyl-phenyl)-4,4-dimethyl-2-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312688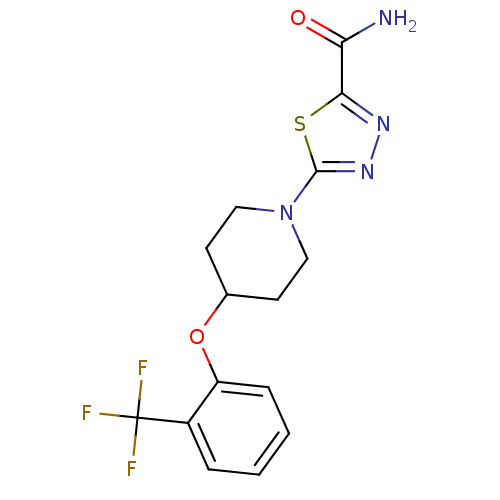 (5-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312684 (2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl)th...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312700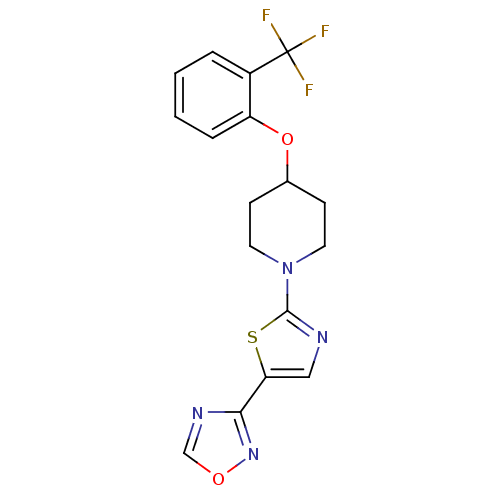 (3-(2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312683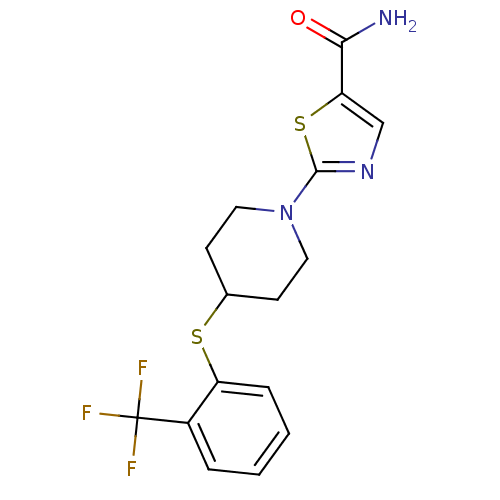 (2-(4-(2-(trifluoromethyl)phenylthio)piperidin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase 5 (Homo sapiens (Human)) | BDBM50364012 (CHEMBL1950397) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD5 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288287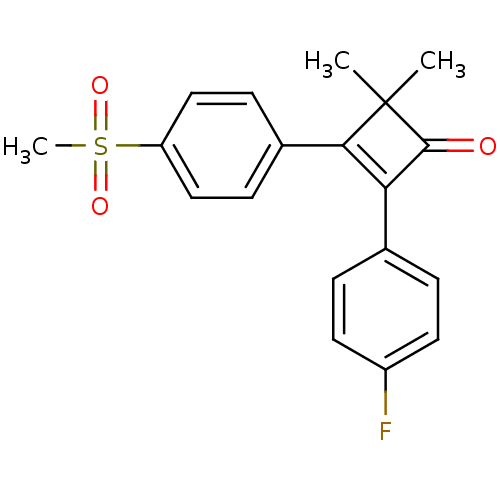 (2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312688 (5-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl)-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50029600 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase 1 was measured by the inhibition of PGE-2 produced by microsomes from U937 cells at subsaturating arachidoni... | Bioorg Med Chem Lett 8: 2777-82 (1999) BindingDB Entry DOI: 10.7270/Q2FX78M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50364012 (CHEMBL1950397) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364013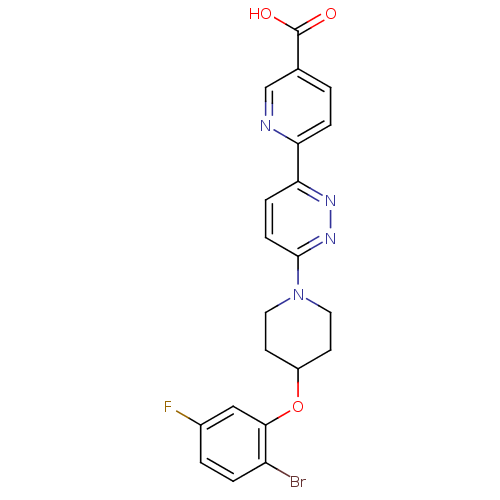 (CHEMBL1950401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312692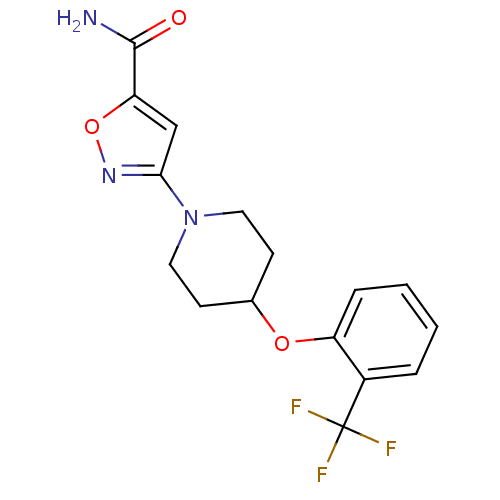 (3-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl)is...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50364012 (CHEMBL1950397) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312684 (2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl)th...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312685 ((S)-2-(3-(2-(trifluoromethyl)phenoxy)pyrrolidin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288294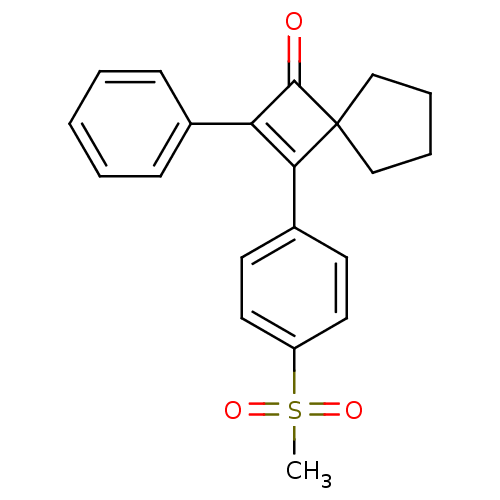 (3-(4-Methanesulfonyl-phenyl)-2-phenyl-spiro[3.4]oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312692 (3-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl)is...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312698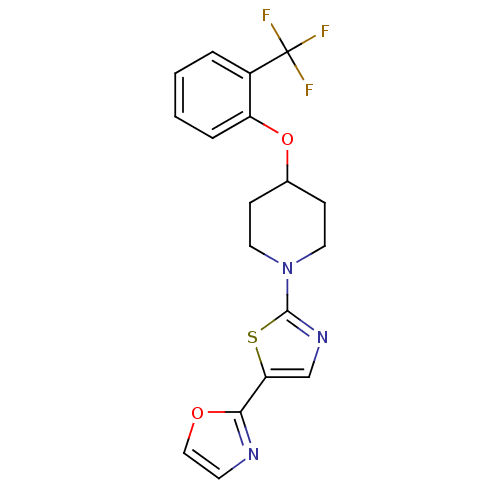 (2-(2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364012 (CHEMBL1950397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364011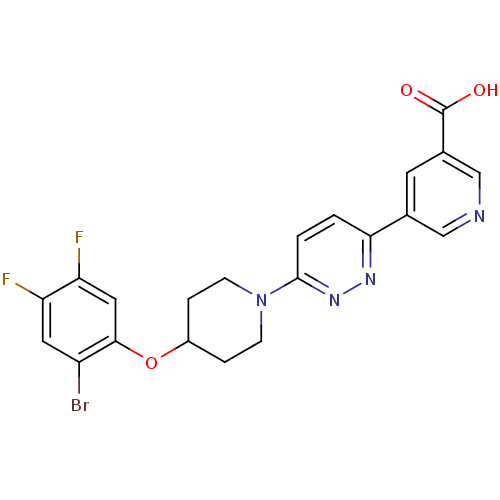 (CHEMBL1950396) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312701 (5-methyl-3-(2-(4-(2-(trifluoromethyl)phenoxy)piper...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312700 (3-(2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50072068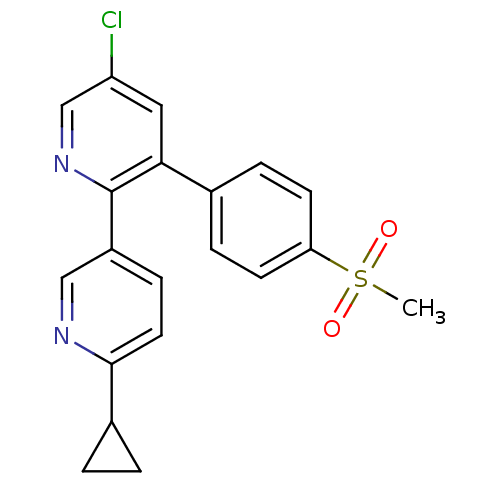 (5-Chloro-6'-cyclopropyl-3-(4-methanesulfonyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of PGE-2 produced by arachidonic acid-stimulated CHO cells stably expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 8: 2777-82 (1999) BindingDB Entry DOI: 10.7270/Q2FX78M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase 1 was measured by the inhibition of PGE-2 produced by microsomes from U937 cells incubated in low concentrat... | Bioorg Med Chem Lett 8: 2777-82 (1999) BindingDB Entry DOI: 10.7270/Q2FX78M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364018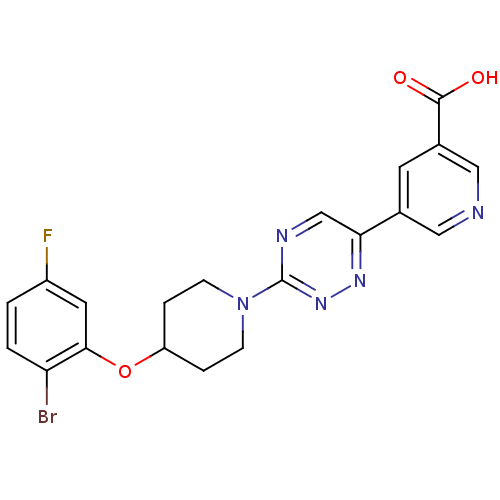 (CHEMBL1950407) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364014 (CHEMBL1950402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50072066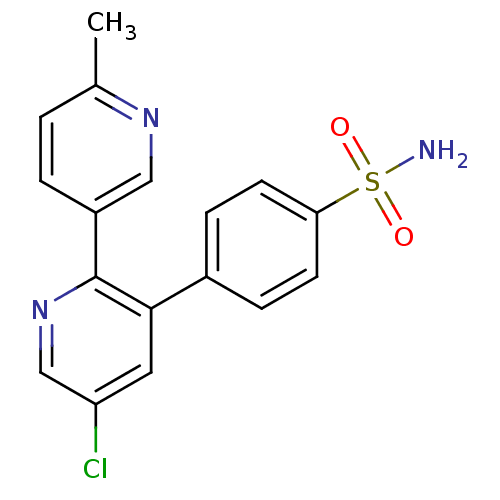 (4-(5-Chloro-6'-methyl-[2,3']bipyridinyl-3-yl)-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of PGE-2 produced by arachidonic acid-stimulated CHO cells stably expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 8: 2777-82 (1999) BindingDB Entry DOI: 10.7270/Q2FX78M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312683 (2-(4-(2-(trifluoromethyl)phenylthio)piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364017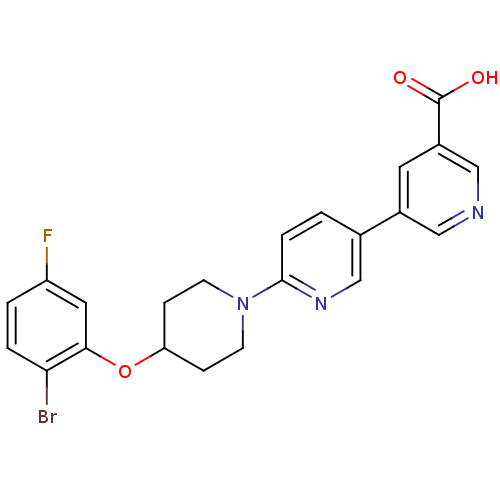 (CHEMBL1950405) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of PGE-2 produced by arachidonic acid-stimulated CHO cells stably expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 8: 2777-82 (1999) BindingDB Entry DOI: 10.7270/Q2FX78M0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364022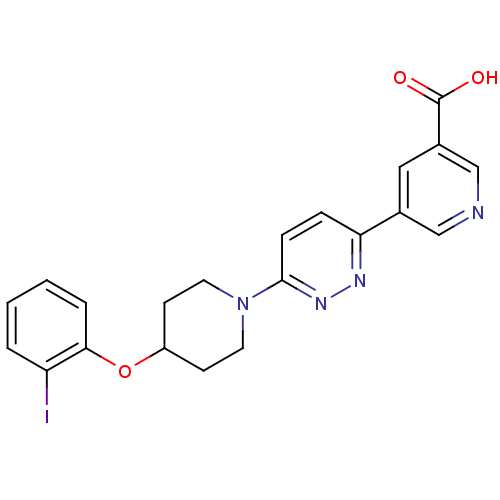 (CHEMBL1950395) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312699 (2-(2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50072067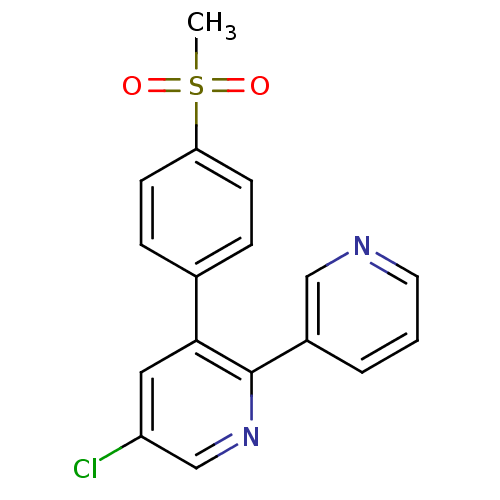 (5-Chloro-3-(4-methanesulfonyl-phenyl)-[2,3']bipyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase 2 was measured by the inhibition of PGE-2 produced by lipopolysaccharide-challenged HWB | Bioorg Med Chem Lett 8: 2777-82 (1999) BindingDB Entry DOI: 10.7270/Q2FX78M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production in arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312701 (5-methyl-3-(2-(4-(2-(trifluoromethyl)phenoxy)piper...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50080083 (3-Cyclohexyloxy-4-(4-methanesulfonyl-phenyl)-5,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay | Bioorg Med Chem Lett 9: 2207-12 (1999) BindingDB Entry DOI: 10.7270/Q2416W8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50080087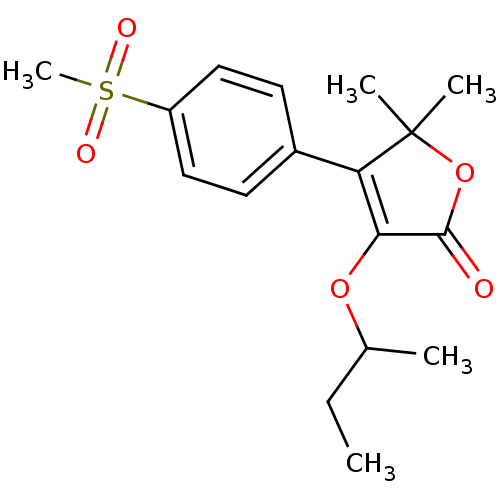 (3-sec-Butoxy-4-(4-methanesulfonyl-phenyl)-5,5-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 9: 2207-12 (1999) BindingDB Entry DOI: 10.7270/Q2416W8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50312693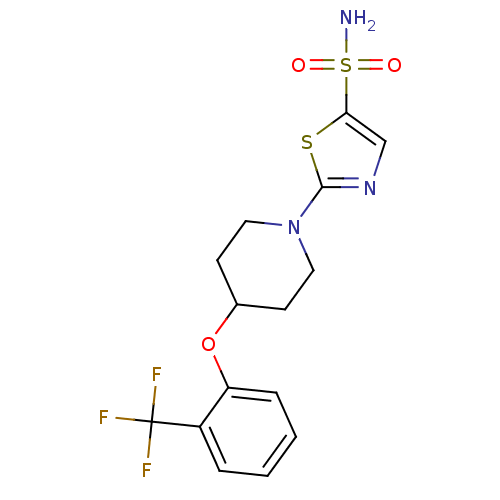 (2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-yl)th...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in Wistar rat liver microsome assessed as [3H]stearoyl-CoA to [3H]oleoyl-CoA conversion pretreated 1 hr before [3H]stearoyl-CoA ad... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50364021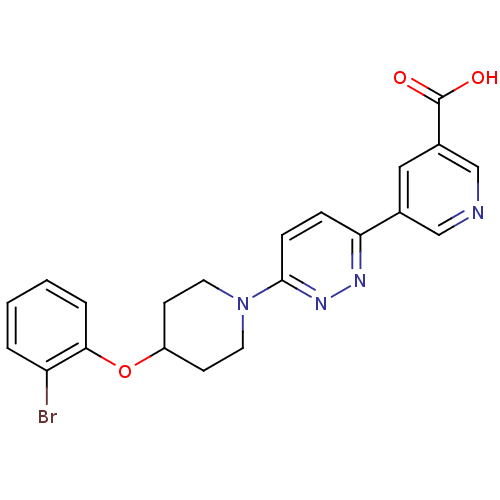 (CHEMBL1950394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD1 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 295 total ) | Next | Last >> |