

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150870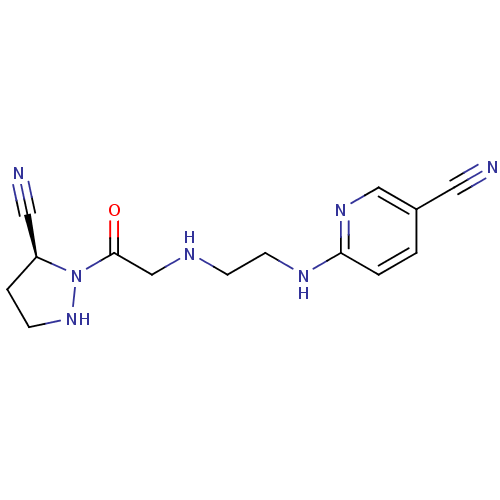 (6-{2-[2-((S)-5-Cyano-pyrazolidin-1-yl)-2-oxo-ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50150851 (2-((R)-2-Amino-3-methyl-pentanoyl)-3,4-dihydro-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against dipeptidyl peptidase IV in Caco-2 cell assay | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50177318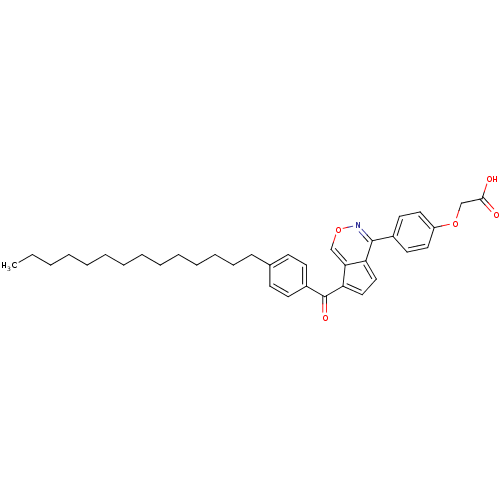 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against cdc25B phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50150851 (2-((R)-2-Amino-3-methyl-pentanoyl)-3,4-dihydro-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against dipeptidyl peptidase IV of porcine kidney | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against CD45 phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50150858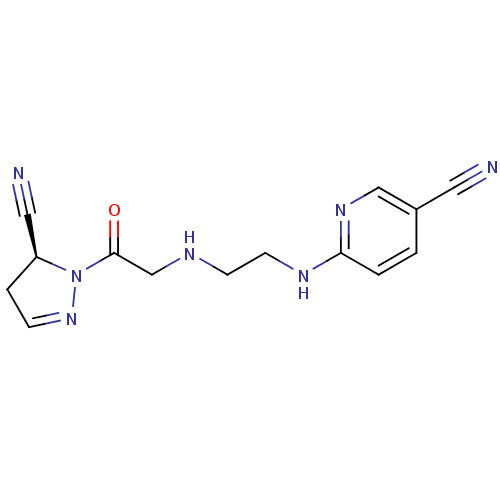 (6-{2-[2-((S)-5-Cyano-4,5-dihydro-pyrazol-1-yl)-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against dipeptidyl peptidase IV in Caco-2 cell assay | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against Protein phosphatase 1 | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150851 (2-((R)-2-Amino-3-methyl-pentanoyl)-3,4-dihydro-2H-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177324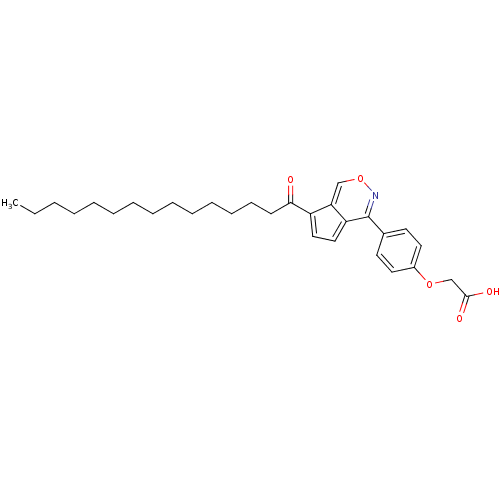 (2-(4-(7-pentadecanoylcyclopenta[d][1,2]oxazin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115725 (3-(1-Indol-1-yl-3,4-dioxo-3,4-dihydro-naphthalen-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115722 (4-Cyclohexyl-[1,2]naphthoquinone | CHEMBL58737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115756 (3-(5,6-Dioxo-8-phenyl-5,6-dihydro-naphthalen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150858 (6-{2-[2-((S)-5-Cyano-4,5-dihydro-pyrazol-1-yl)-2-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50150854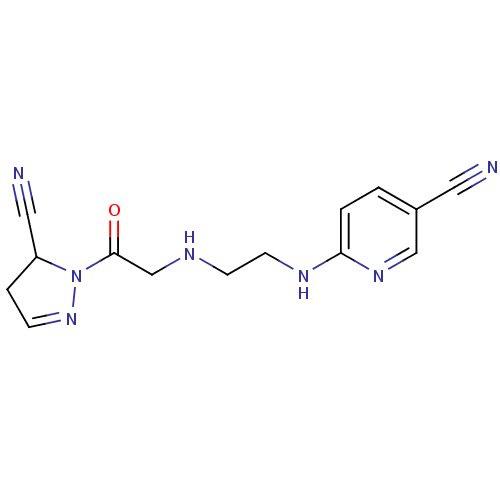 (6-(2-(2-(5-cyano-4,5-dihydro-1H-pyrazol-1-yl)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against dipeptidyl peptidase IV in Caco-2 cell assay | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115733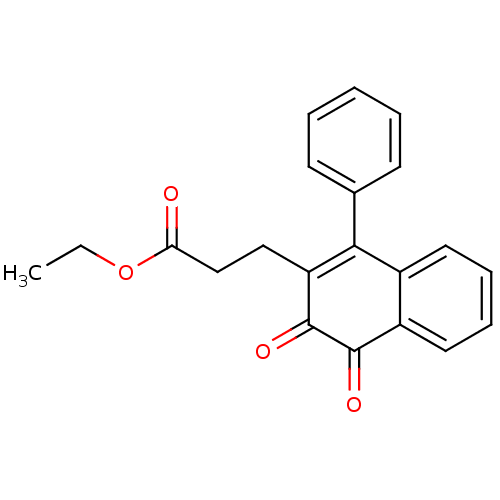 (3-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50150858 (6-{2-[2-((S)-5-Cyano-4,5-dihydro-pyrazol-1-yl)-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against dipeptidyl peptidase IV of porcine kidney | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115766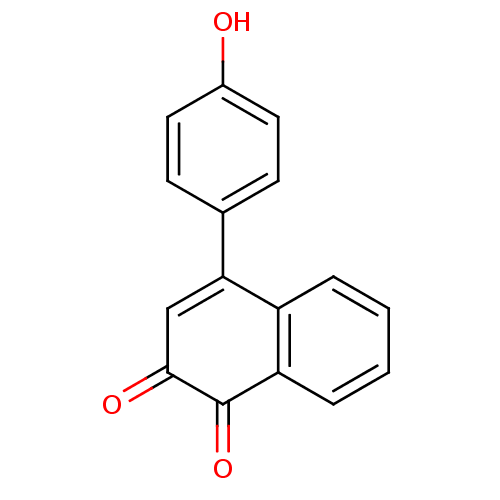 (4-(4-Hydroxy-phenyl)-[1,2]naphthoquinone | CHEMBL5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115760 (4-(2,5-Difluoro-phenyl)-[1,2]naphthoquinone | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase YopH (Yersinia enterocolitica) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against YOP protein tyrosine phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115721 (3-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115740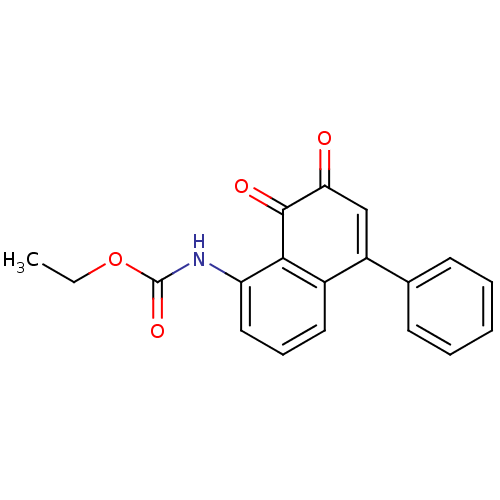 ((7,8-Dioxo-5-phenyl-7,8-dihydro-naphthalen-1-yl)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115739 (CHEMBL56948 | [4-(1-Indol-1-yl-3,4-dioxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150860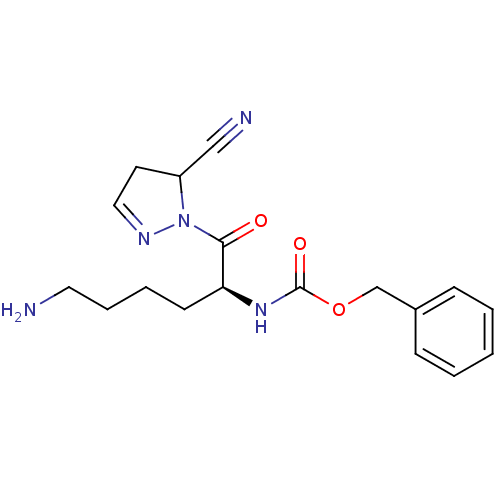 (CHEMBL361656 | [(S)-5-Amino-1-(5-cyano-4,5-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115746 (3-[7-(2-Methoxycarbonyl-ethyl)-3,4-dioxo-1-phenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against LAR protein tyrosine phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150854 (6-(2-(2-(5-cyano-4,5-dihydro-1H-pyrazol-1-yl)-2-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177317 (2-(4-(7-(4-methoxybenzoyl)cyclopenta[d][1,2]oxazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099778 (4-Phenyl-[1,2]naphthoquinone | CHEMBL51447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant human CD45 using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50099778 (4-Phenyl-[1,2]naphthoquinone | CHEMBL51447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115763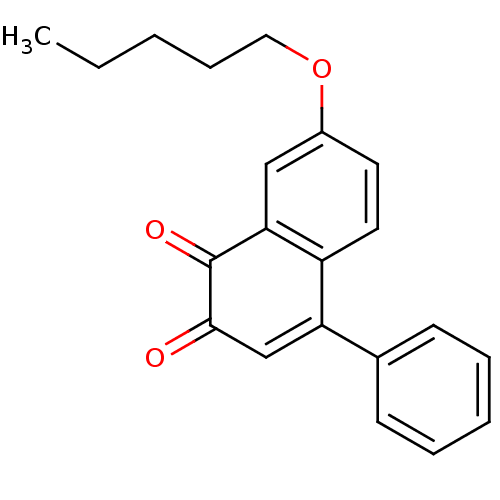 (7-Pentyloxy-4-phenyl-[1,2]naphthoquinone | CHEMBL5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115755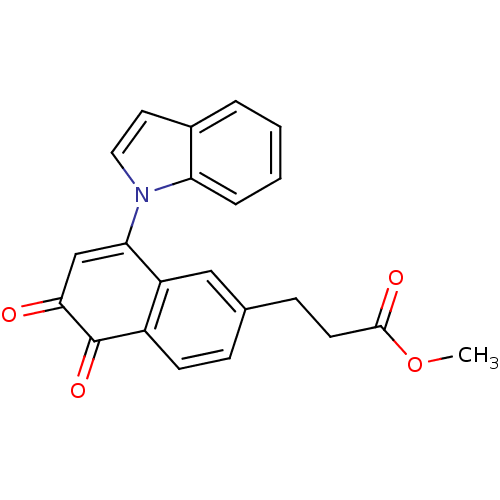 (3-(8-Indol-1-yl-5,6-dioxo-5,6-dihydro-naphthalen-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115751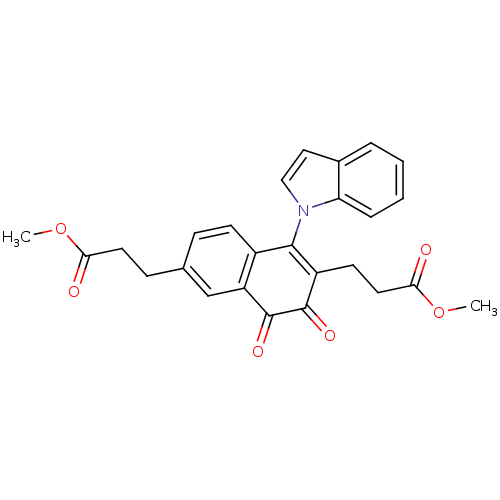 (3-[1-Indol-1-yl-6-(2-methoxycarbonyl-ethyl)-3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50150854 (6-(2-(2-(5-cyano-4,5-dihydro-1H-pyrazol-1-yl)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against dipeptidyl peptidase IV of porcine kidney | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115728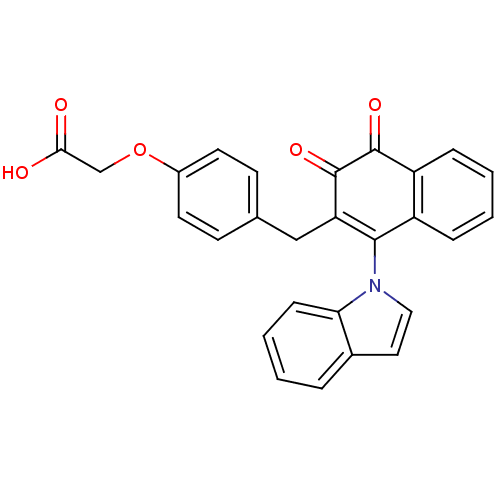 (CHEMBL57841 | [4-(1-Indol-1-yl-3,4-dioxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115761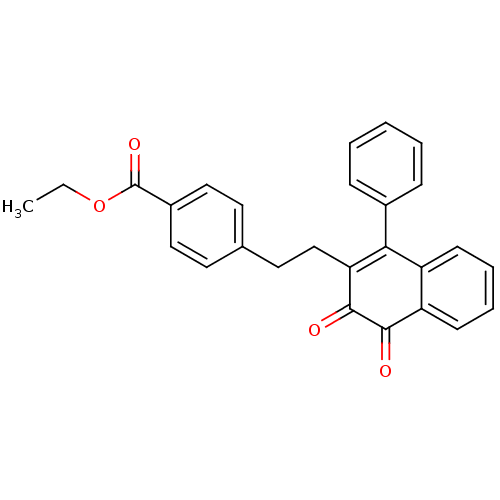 (4-[2-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115729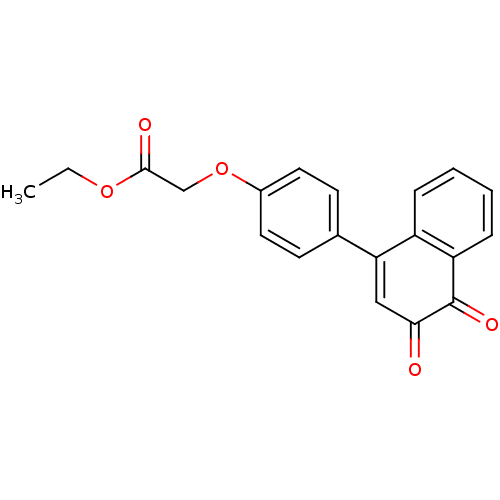 (CHEMBL292843 | [4-(3,4-Dioxo-3,4-dihydro-naphthale...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115737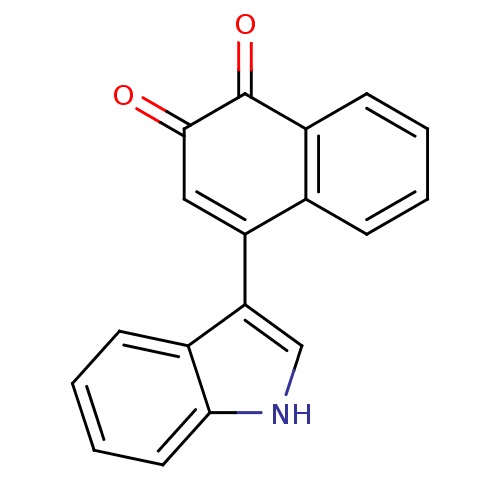 (4-(1H-Indol-3-yl)-[1,2]naphthoquinone | CHEMBL6070...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115727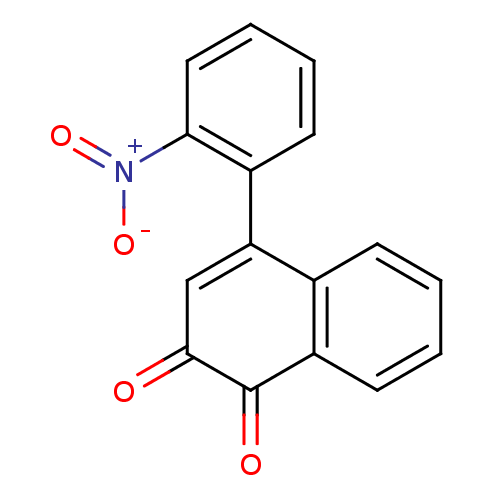 (4-(2-Nitro-phenyl)-[1,2]naphthoquinone | CHEMBL592...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase YopH (Yersinia enterocolitica) | BDBM50177316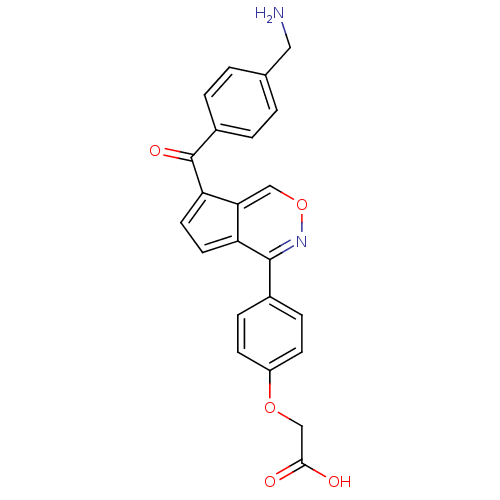 (2-(4-(7-(4-(aminomethyl)benzoyl)cyclopenta[d][1,2]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against YOP protein tyrosine phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150865 (2-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150862 (2-((R)-2-Amino-propionyl)-3,4-dihydro-2H-pyrazole-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150866 (2-{2-[4-(5-Nitro-pyridin-2-ylamino)-cyclohexylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50177316 (2-(4-(7-(4-(aminomethyl)benzoyl)cyclopenta[d][1,2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against CD45 phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50177316 (2-(4-(7-(4-(aminomethyl)benzoyl)cyclopenta[d][1,2]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against Protein phosphatase 1 | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50150856 (2-(2-Cyclopentylamino-acetyl)-3,4-dihydro-2H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory concentration against rat dipeptidyl peptidase IV | Bioorg Med Chem Lett 14: 4461-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.046 BindingDB Entry DOI: 10.7270/Q2J9674N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against cdc25A phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115744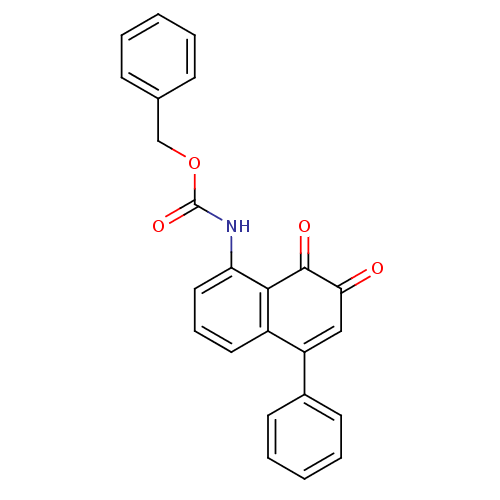 ((7,8-Dioxo-5-phenyl-7,8-dihydro-naphthalen-1-yl)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115765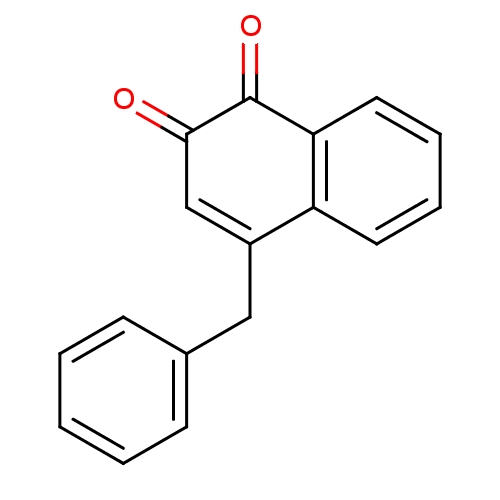 (4-Benzyl-[1,2]naphthoquinone | CHEMBL292386) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115735 (CHEMBL58354 | [4-(3,4-Dioxo-1-phenyl-3,4-dihydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |