

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212293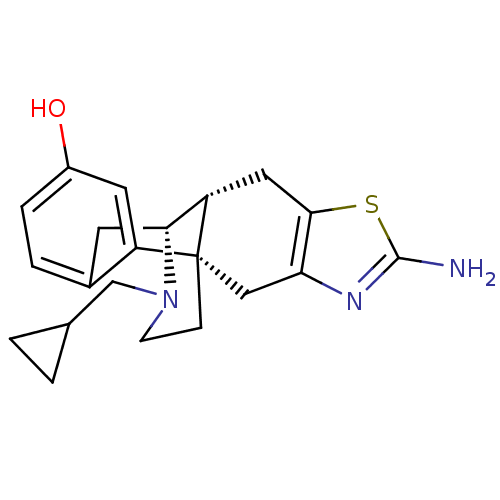 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212297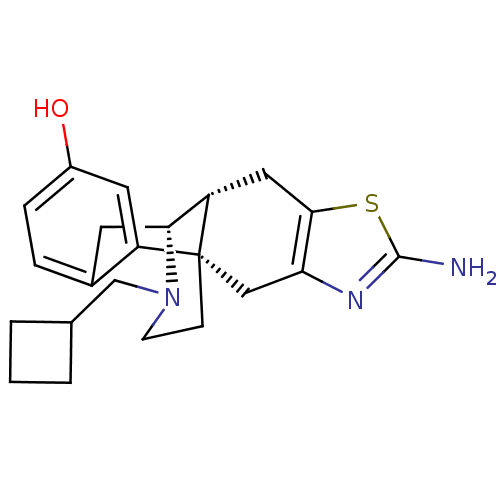 ((1S,9R,10R)-5-amino-20-(cyclobutylmethyl)-6-thia-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212291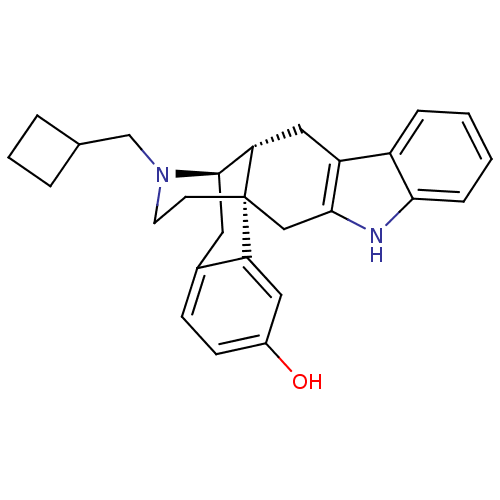 ((1S,13R,14R)-24-(cyclobutylmethyl)-4,24-diazahexac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212296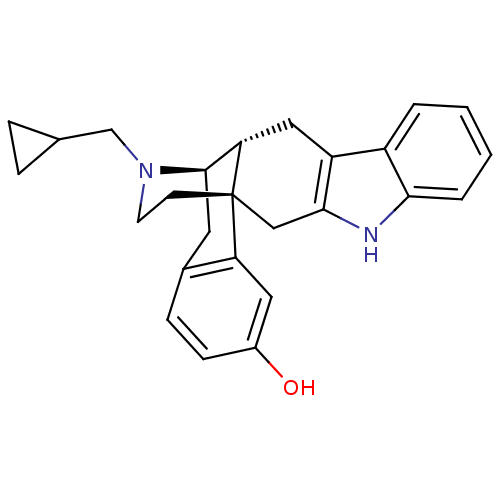 ((1S,13R,14R)-24-(cyclopropylmethyl)-4,24-diazahexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212295 ((1S,13R,14R)-24-methyl-4,24-diazahexacyclo[12.7.3....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212297 ((1S,9R,10R)-5-amino-20-(cyclobutylmethyl)-6-thia-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212299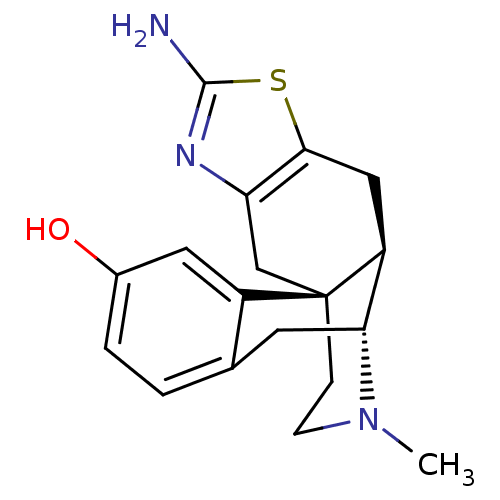 ((1S,9R,10R)-5-amino-20-methyl-6-thia-4,20-diazapen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212296 ((1S,13R,14R)-24-(cyclopropylmethyl)-4,24-diazahexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212291 ((1S,13R,14R)-24-(cyclobutylmethyl)-4,24-diazahexac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212297 ((1S,9R,10R)-5-amino-20-(cyclobutylmethyl)-6-thia-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212296 ((1S,13R,14R)-24-(cyclopropylmethyl)-4,24-diazahexa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212292 ((1S,13R,14R)-24-(cyclopropylmethyl)-19-methoxy-4,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212292 ((1S,13R,14R)-24-(cyclopropylmethyl)-19-methoxy-4,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212299 ((1S,9R,10R)-5-amino-20-methyl-6-thia-4,20-diazapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212291 ((1S,13R,14R)-24-(cyclobutylmethyl)-4,24-diazahexac...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212299 ((1S,9R,10R)-5-amino-20-methyl-6-thia-4,20-diazapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212292 ((1S,13R,14R)-24-(cyclopropylmethyl)-19-methoxy-4,2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212298 ((1S,13R,14R)-24-(cyclobutylmethyl)-19-methoxy-4,24...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50088341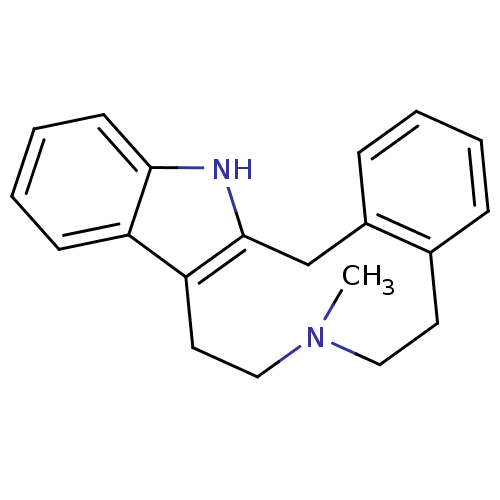 (11-methyl-11,21-diazatetracyclo[12.7.0.0^{3,8}.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Drug Research Curated by ChEMBL | Assay Description Antagonist activity at dopamine D5 receptor (unknown origin) | ACS Med Chem Lett 5: 304-8 (2014) Article DOI: 10.1021/ml400373j BindingDB Entry DOI: 10.7270/Q2CN75F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50088341 (11-methyl-11,21-diazatetracyclo[12.7.0.0^{3,8}.0^{...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Drug Research Curated by ChEMBL | Assay Description Antagonist activity at dopamine D1 receptor (unknown origin) | ACS Med Chem Lett 5: 304-8 (2014) Article DOI: 10.1021/ml400373j BindingDB Entry DOI: 10.7270/Q2CN75F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212295 ((1S,13R,14R)-24-methyl-4,24-diazahexacyclo[12.7.3....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212295 ((1S,13R,14R)-24-methyl-4,24-diazahexacyclo[12.7.3....) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212294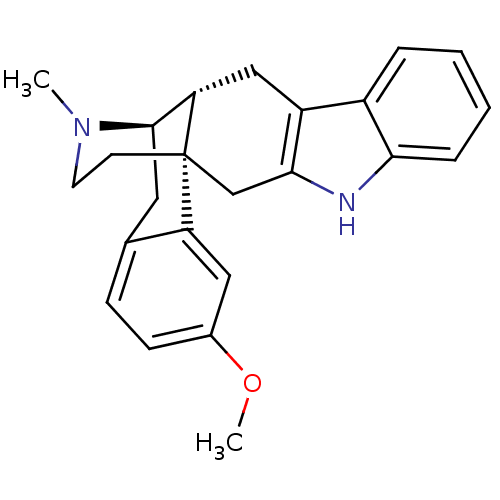 ((1S,13R,14R)-19-methoxy-24-methyl-4,24-diazahexacy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50407504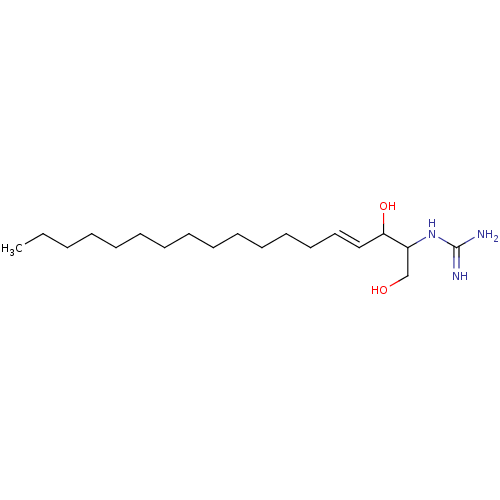 (CHEMBL5275740) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (Alpha-1A adrenergic receptor ) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004822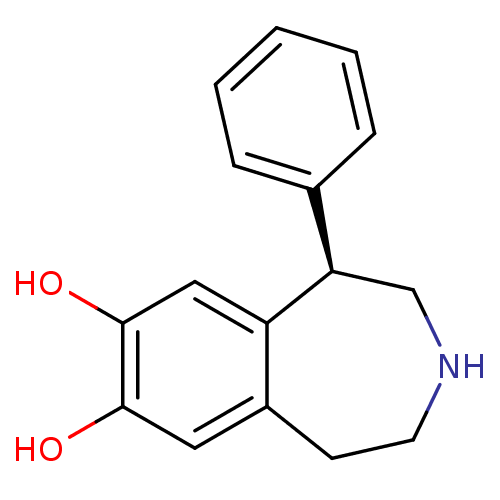 ((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Drug Research Curated by ChEMBL | Assay Description Agonist activity at dopamine D1 receptor (unknown origin) | ACS Med Chem Lett 5: 304-8 (2014) Article DOI: 10.1021/ml400373j BindingDB Entry DOI: 10.7270/Q2CN75F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212298 ((1S,13R,14R)-24-(cyclobutylmethyl)-19-methoxy-4,24...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50407504 (CHEMBL5275740) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of the cAMP-stimulated beta-galactosidase transcription in SK-N-MC cells expressing the human Histamine H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50600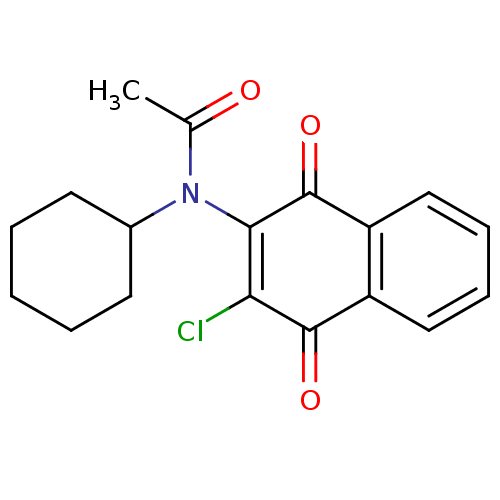 (MLS001029919 | N-(3-Chloro-1,4-dioxo-1,4-dihydro-n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212298 ((1S,13R,14R)-24-(cyclobutylmethyl)-19-methoxy-4,24...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212294 ((1S,13R,14R)-19-methoxy-24-methyl-4,24-diazahexacy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212294 ((1S,13R,14R)-19-methoxy-24-methyl-4,24-diazahexacy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Mus musculus (Mouse)) | BDBM50407506 (CHEMBL4595333) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Mus musculus (Mouse)) | BDBM50407507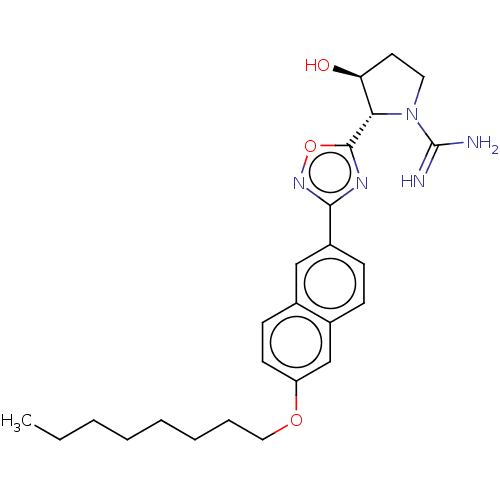 (CHEMBL5281819) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50443388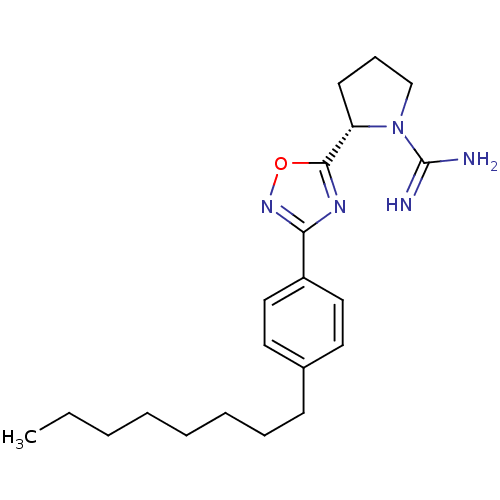 (CHEMBL3086782 | US9688668, SLR080811) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50009730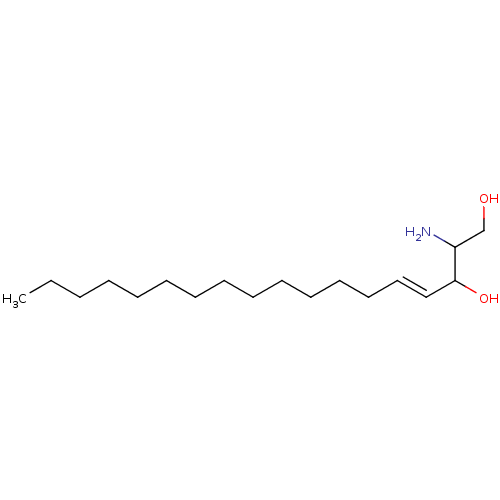 ((E)-2-Amino-octadec-4-ene-1,3-diol | 2-Amino-octad...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat prostatic vas deferens (alpha1A receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50407505 (CHEMBL447685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-phenylephrine-induced contraction of rat spleen (Alpha-1B adrenergic receptor ) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50139649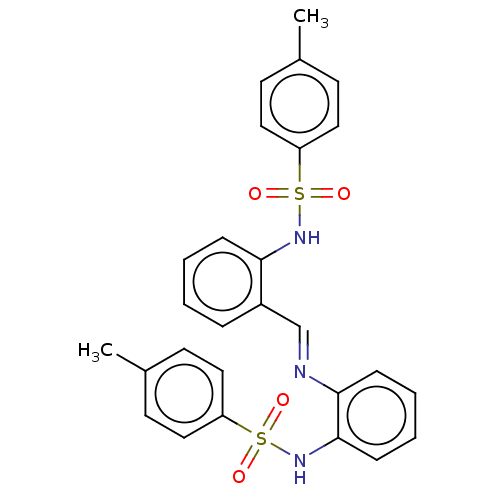 (CHEMBL3764617) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50393642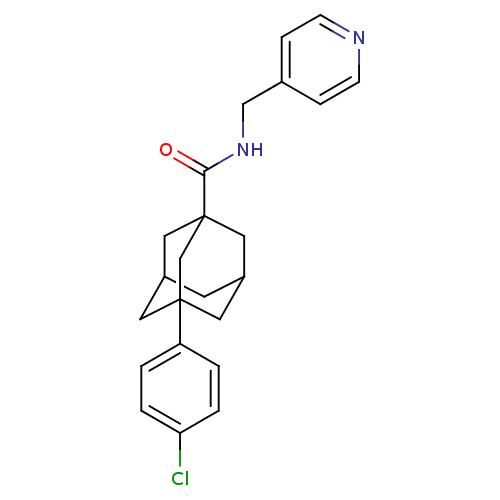 (CHEMBL2158685) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50443388 (CHEMBL3086782 | US9688668, SLR080811) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50407505 (CHEMBL447685) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Mus musculus) | BDBM50407506 (CHEMBL4595333) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Mus musculus) | BDBM50407507 (CHEMBL5281819) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50139649 (CHEMBL3764617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against (-)-noradrenaline-induced contraction of rat thoracic aorta (Alpha-1D adrenergic receptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212291 ((1S,13R,14R)-24-(cyclobutylmethyl)-4,24-diazahexac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO membrane assessed as inhibition of SNC80-induced [35S]GTPgammaS binding | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50499123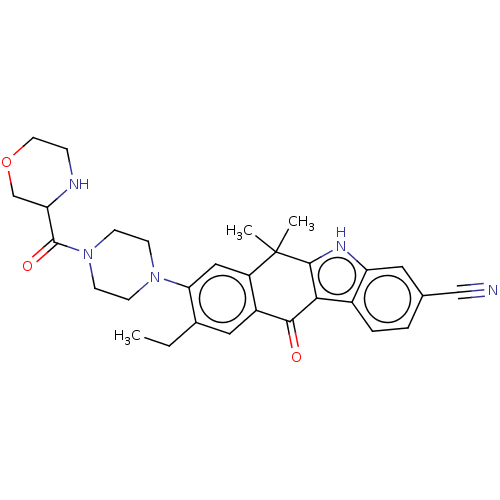 (CHEMBL3734938) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant ALK (unknown origin) using poly (Glu, Tyr)4:1 substrate incubated for 60 mins by ELISA | Eur J Med Chem 105: 39-56 (2015) Article DOI: 10.1016/j.ejmech.2015.10.005 BindingDB Entry DOI: 10.7270/Q2BV7KM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50499104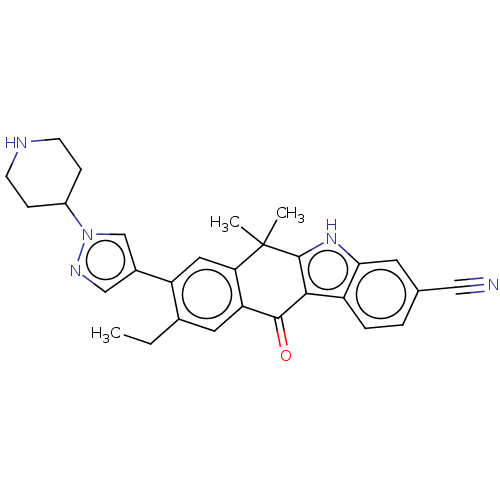 (CHEMBL3736273) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant ALK (unknown origin) using poly (Glu, Tyr)4:1 substrate incubated for 60 mins by ELISA | Eur J Med Chem 105: 39-56 (2015) Article DOI: 10.1016/j.ejmech.2015.10.005 BindingDB Entry DOI: 10.7270/Q2BV7KM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 521 total ) | Next | Last >> |