 Found 82 hits with Last Name = 'yeung' and Initial = 'bk'
Found 82 hits with Last Name = 'yeung' and Initial = 'bk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18793
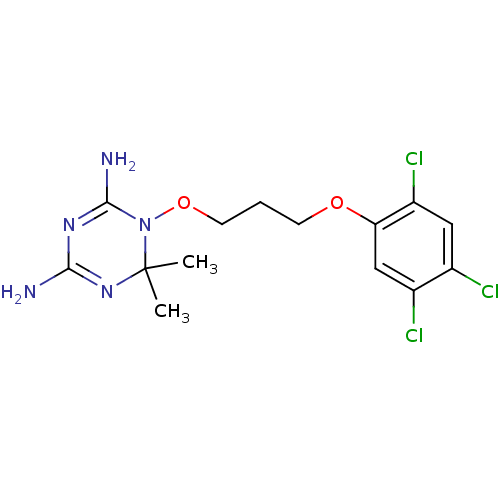
(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50035483
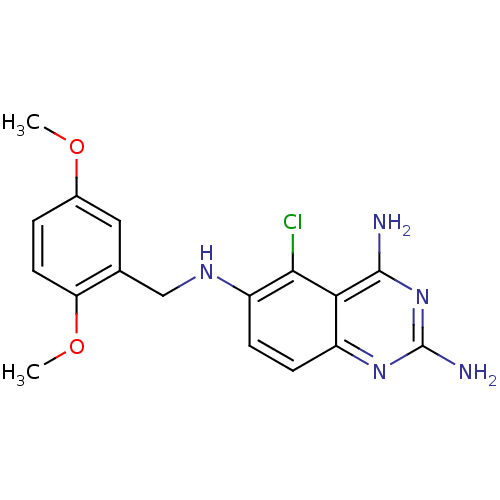
(5-Chloro-N*6*-(2,5-dimethoxy-benzyl)-quinazoline-2...)Show InChI InChI=1S/C17H18ClN5O2/c1-24-10-3-6-13(25-2)9(7-10)8-21-12-5-4-11-14(15(12)18)16(19)23-17(20)22-11/h3-7,21H,8H2,1-2H3,(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18512
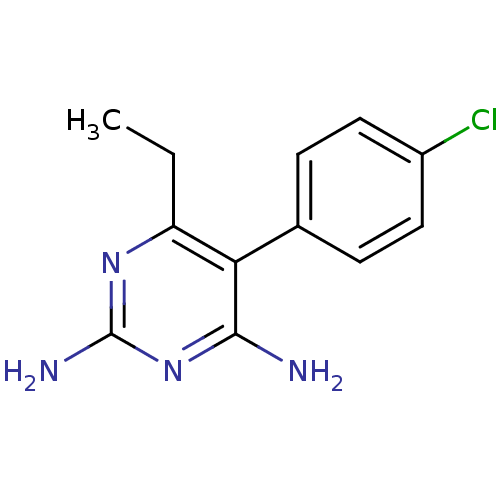
(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18792
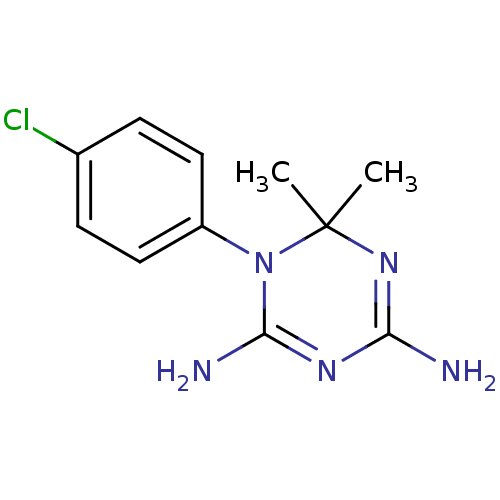
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 55.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318672
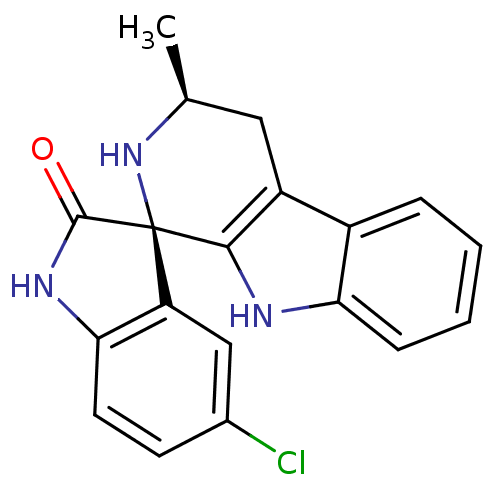
((1R,3S)-5'-Chloro-3-methyl-2,3,4,9-tetrahydrospiro...)Show SMILES C[C@H]1Cc2c([nH]c3ccccc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H16ClN3O/c1-10-8-13-12-4-2-3-5-15(12)21-17(13)19(23-10)14-9-11(20)6-7-16(14)22-18(19)24/h2-7,9-10,21,23H,8H2,1H3,(H,22,24)/t10-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318670
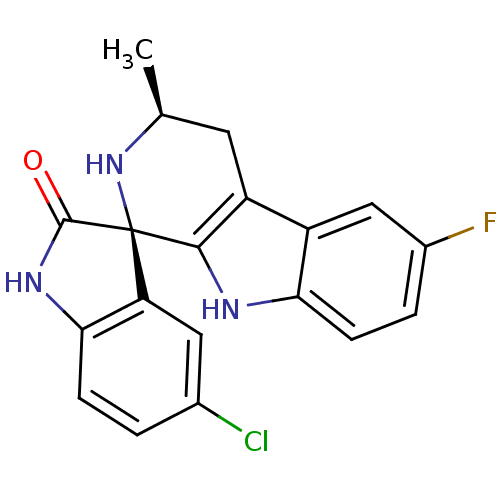
((1R,3S)-5'-Chloro-6-fluoro-3-methyl-2,3,4,9-tetrah...)Show SMILES C[C@H]1Cc2c([nH]c3ccc(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H15ClFN3O/c1-9-6-13-12-8-11(21)3-5-15(12)22-17(13)19(24-9)14-7-10(20)2-4-16(14)23-18(19)25/h2-5,7-9,22,24H,6H2,1H3,(H,23,25)/t9-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318668
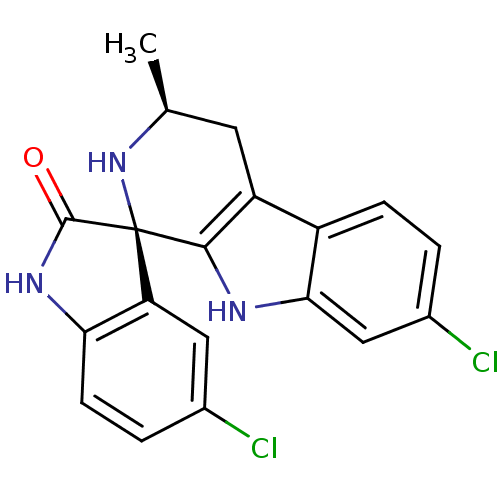
((1R,3S)-5',7-Dichloro-3-methyl-2,3,4,9-tetrahydros...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)ccc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H15Cl2N3O/c1-9-6-13-12-4-2-11(21)8-16(12)22-17(13)19(24-9)14-7-10(20)3-5-15(14)23-18(19)25/h2-5,7-9,22,24H,6H2,1H3,(H,23,25)/t9-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318664
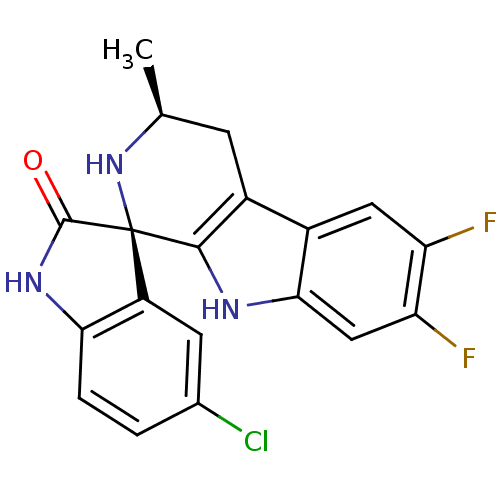
((1R,3S)-5'-Chloro-6,7-difluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(F)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14ClF2N3O/c1-8-4-11-10-6-13(21)14(22)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine 3 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant dopamine transporter |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant histamine H1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant histamine H2 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant histamine H3 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant MAOM |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant motilin receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant muscarinic M1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant muscarinic M2 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant muscarinic M3 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant neurokinin NK1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant neuropeptide Y1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant neuropeptide Y2 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant neurotensin NT1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant niacin receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant norepinephrine transporter |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant opiate kappa receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant opiate mu receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant PDE4D |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant serotonin 5-HT1A receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant serotonin 5-HT2A receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant serotonin 5-HT2B receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant serotonin 5HT2C receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant serotonin 5HT3 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant serotonin transporter |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant vasopressin V1a receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318663
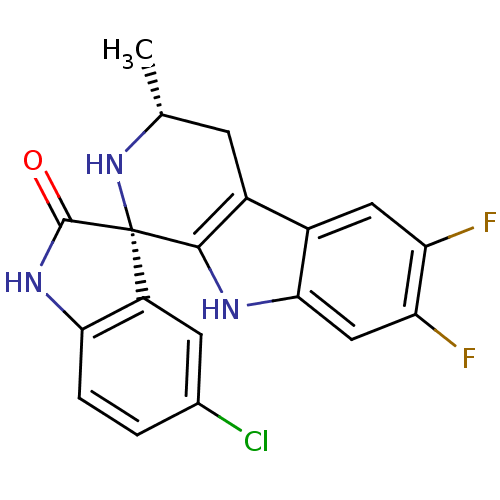
((1S,3R)-5'-Chloro-6,7-difluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@@H]1Cc2c([nH]c3cc(F)c(F)cc23)[C@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14ClF2N3O/c1-8-4-11-10-6-13(21)14(22)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318665
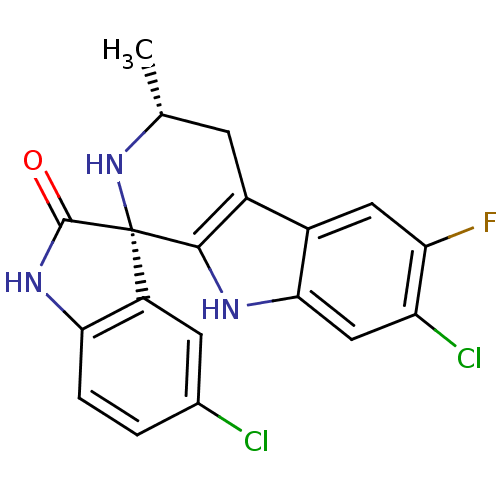
((1S,3R)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318667
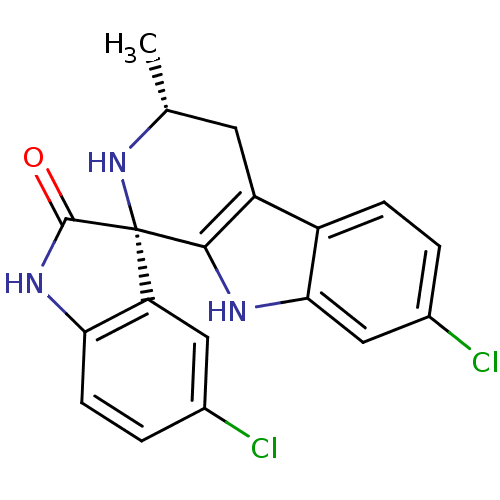
((1S,3R)-5',7-Dichloro-3-methyl-2,3,4,9-tetrahydros...)Show SMILES C[C@@H]1Cc2c([nH]c3cc(Cl)ccc23)[C@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H15Cl2N3O/c1-9-6-13-12-4-2-11(21)8-16(12)22-17(13)19(24-9)14-7-10(20)3-5-15(14)23-18(19)25/h2-5,7-9,22,24H,6H2,1H3,(H,23,25)/t9-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318669
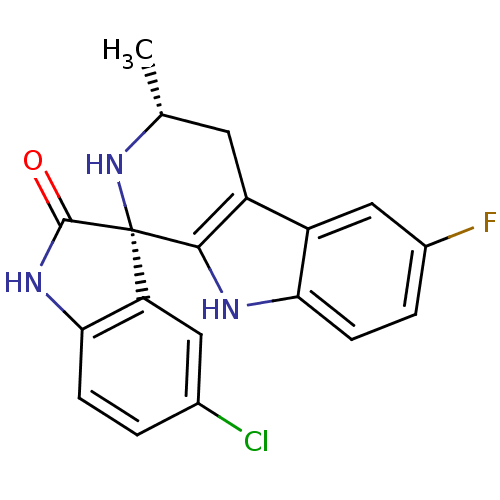
((1S,3R)-5'-Chloro-6-fluoro-3-methyl-2,3,4,9-tetrah...)Show SMILES C[C@@H]1Cc2c([nH]c3ccc(F)cc23)[C@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H15ClFN3O/c1-9-6-13-12-8-11(21)3-5-15(12)22-17(13)19(24-9)14-7-10(20)2-4-16(14)23-18(19)25/h2-5,7-9,22,24H,6H2,1H3,(H,23,25)/t9-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318671
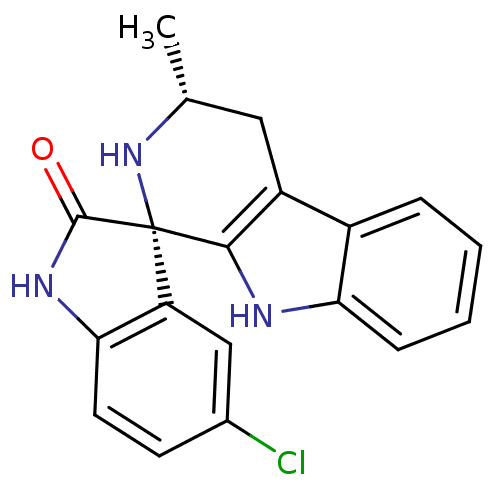
((1S,3R)-5'-Chloro-3-methyl-2,3,4,9-tetrahydrospiro...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H16ClN3O/c1-10-8-13-12-4-2-3-5-15(12)21-17(13)19(23-10)14-9-11(20)6-7-16(14)22-18(19)24/h2-7,9-10,21,23H,8H2,1H3,(H,22,24)/t10-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome by LC-MS/MS analysis |
J Med Chem 53: 5155-64 (2010)
Article DOI: 10.1021/jm100410f
BindingDB Entry DOI: 10.7270/Q2TM7C2C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant dopamine D3 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant dopamine D2 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant dopamine D1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant cannabinoid 1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant alpha2c receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant alpha-2a receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant alpha 1a receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adrenergic beta-1 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant alpha2b receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318666

((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...)Show SMILES C[C@H]1Cc2c([nH]c3cc(Cl)c(F)cc23)[C@@]2(N1)C(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adrenergic beta2 receptor |
Science 329: 1175-80 (2010)
Article DOI: 10.1126/science.1193225
BindingDB Entry DOI: 10.7270/Q2SB45ZB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data