

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of [3H]- DPCPX binding to Adenosine A1 receptor ofrat brain membranes | J Med Chem 41: 2676-8 (1998) Article DOI: 10.1021/jm9802822 BindingDB Entry DOI: 10.7270/Q2NC61WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of [3H]- CGS 21680 binding to Adenosine A2A receptor of rat brain membranes | J Med Chem 41: 2676-8 (1998) Article DOI: 10.1021/jm9802822 BindingDB Entry DOI: 10.7270/Q2NC61WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50369378 (CHEMBL606438) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of [3H]- DPCPX binding to Adenosine A1 receptor ofrat brain membranes | J Med Chem 41: 2676-8 (1998) Article DOI: 10.1021/jm9802822 BindingDB Entry DOI: 10.7270/Q2NC61WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455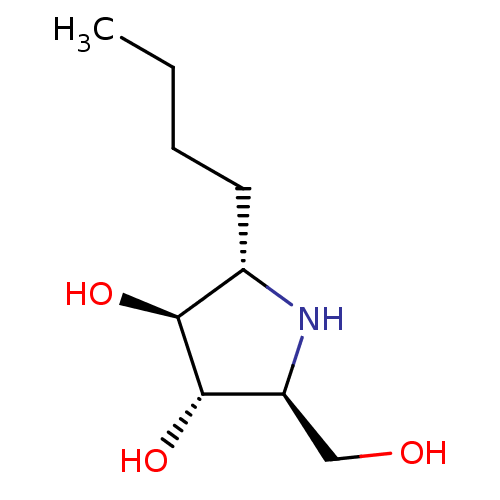 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of [125I]AB-MECA (0.15 nM) binding to Adenosine A3 receptor of RBL-2H3 cell membranes | J Med Chem 41: 2676-8 (1998) Article DOI: 10.1021/jm9802822 BindingDB Entry DOI: 10.7270/Q2NC61WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50242271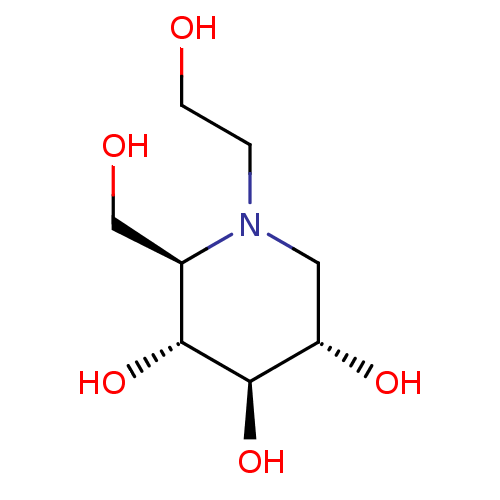 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Activity against Adenosine A2B receptor as cAMP production in VA-13 cells | J Med Chem 41: 2676-8 (1998) Article DOI: 10.1021/jm9802822 BindingDB Entry DOI: 10.7270/Q2NC61WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389789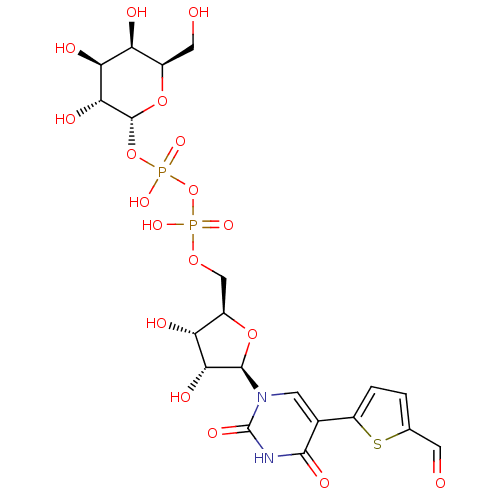 (CHEMBL1229737) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389786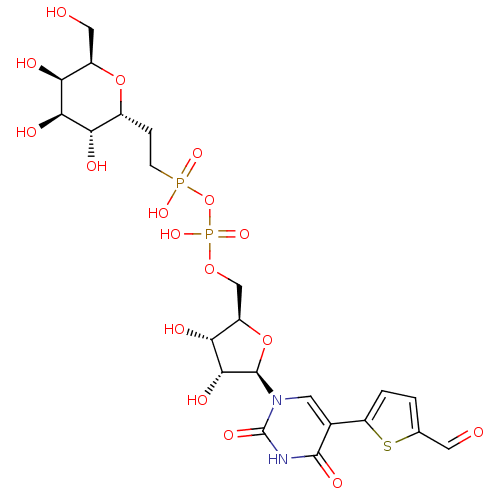 (CHEMBL2070378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389788 (CHEMBL2070375) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Bos taurus) | BDBM50389787 (CHEMBL2070376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of East Anglia Curated by ChEMBL | Assay Description Inhibition of alpha-1,3-GalT in bovine after 15 mins by Dixon plot analysis | J Med Chem 55: 2015-24 (2012) Article DOI: 10.1021/jm201154p BindingDB Entry DOI: 10.7270/Q28W3FCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048090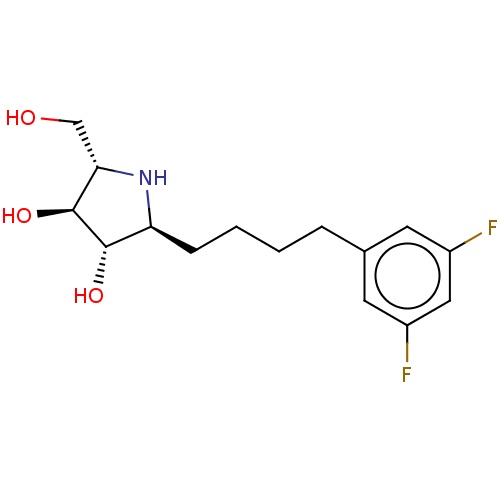 (CHEMBL3311519) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine sucrase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048086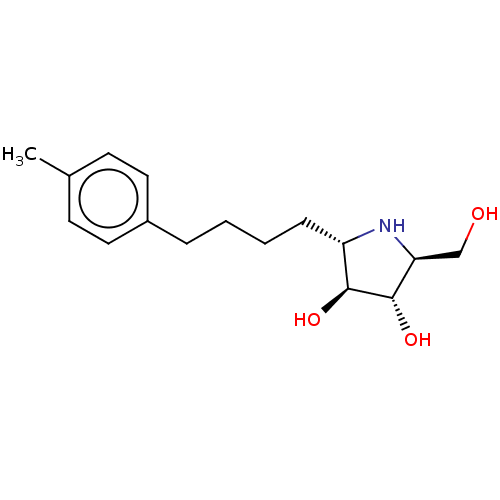 (CHEMBL3311515) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801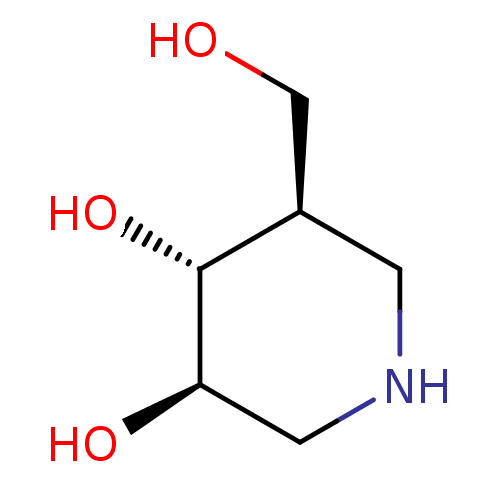 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human lysosome beta-glucosidase assessed as production of 4-methylumbelliferone using 4-methylumbelliferyl beta-D-glucoside as substrat... | Bioorg Med Chem 19: 3558-68 (2011) Article DOI: 10.1016/j.bmc.2011.04.011 BindingDB Entry DOI: 10.7270/Q2BC3ZWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333457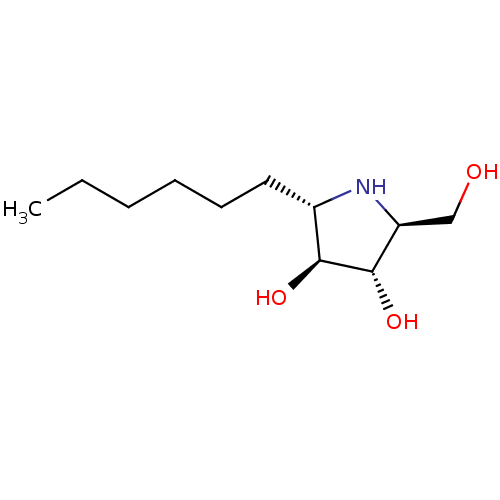 ((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine sucrase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333457 ((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048088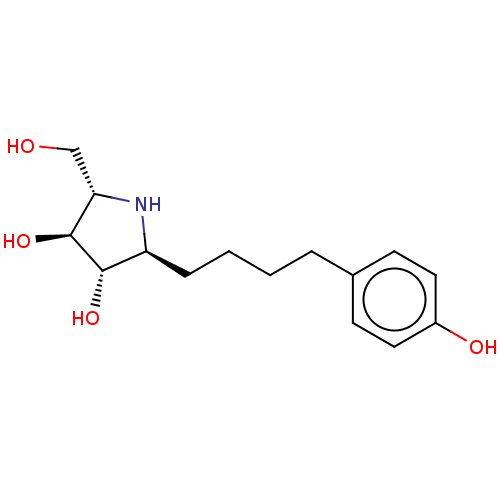 (CHEMBL3311517) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50048087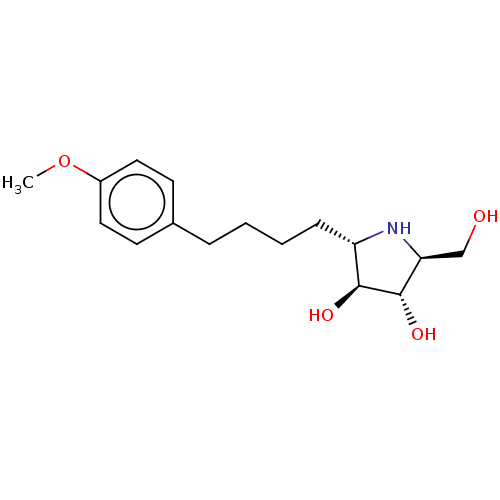 (CHEMBL3311516) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18363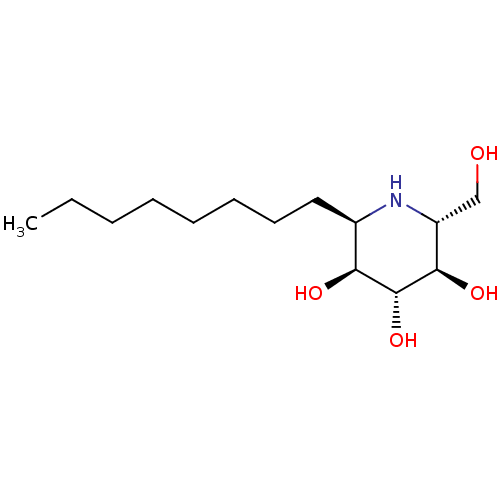 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044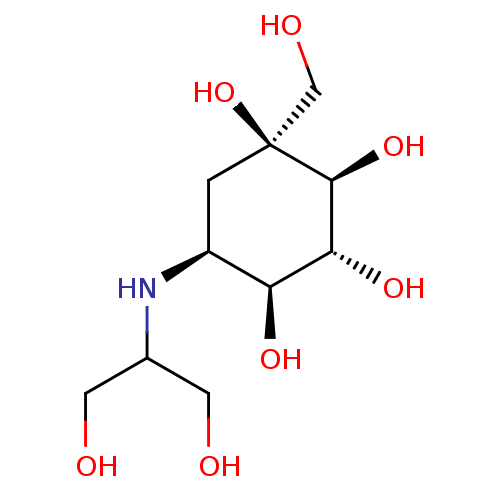 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactase/phlorizin hydrolase (Rattus norvegicus) | BDBM50182801 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal lactase assessed as production of p-nitrophenol by spectrophotometry | Bioorg Med Chem 19: 3558-68 (2011) Article DOI: 10.1016/j.bmc.2011.04.011 BindingDB Entry DOI: 10.7270/Q2BC3ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048087 (CHEMBL3311516) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333465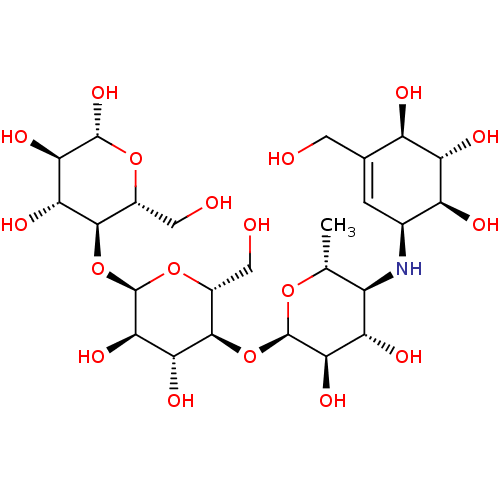 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine maltase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048089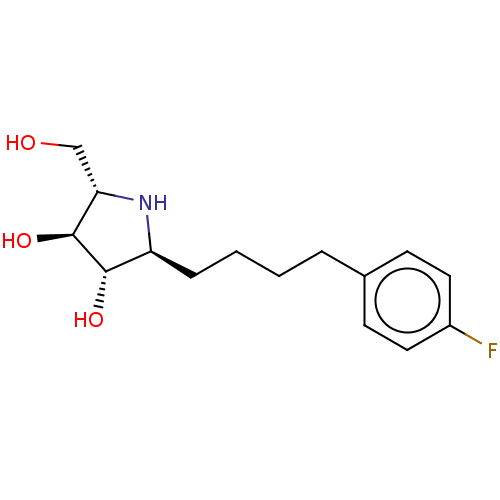 (CHEMBL3311518) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50048088 (CHEMBL3311517) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333465 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine maltase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50048089 (CHEMBL3311518) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333465 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333456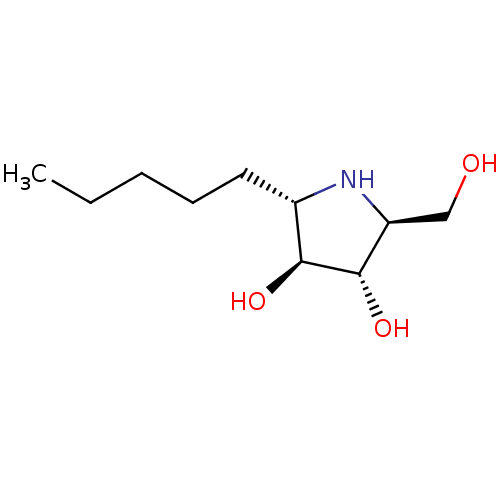 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-pentylpyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50399654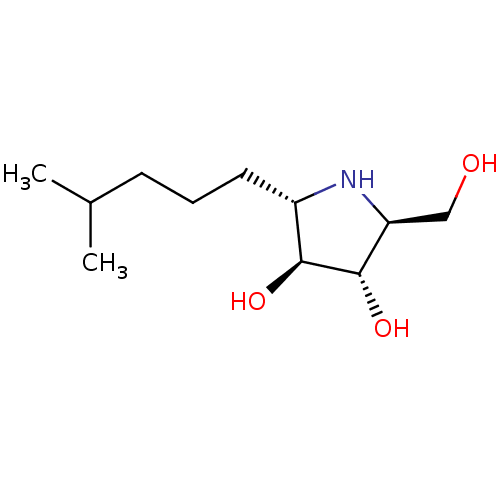 (CHEMBL2177691) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333456 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-pentylpyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine sucrase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine maltase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50399655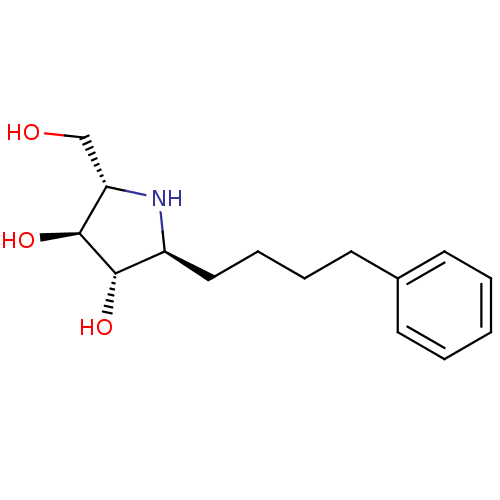 (CHEMBL2177690) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50048090 (CHEMBL3311519) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal isomaltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50399655 (CHEMBL2177690) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333458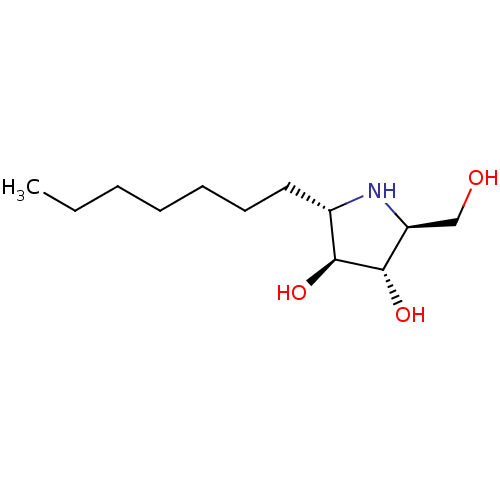 ((2S,3S,4S,5S)-2-heptyl-5-(hydroxymethyl)pyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine sucrase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50399654 (CHEMBL2177691) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333458 ((2S,3S,4S,5S)-2-heptyl-5-(hydroxymethyl)pyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333454 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-propylpyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333454 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-propylpyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat small intestine sucrase after 30 mins | Bioorg Med Chem Lett 21: 738-41 (2011) Article DOI: 10.1016/j.bmcl.2010.11.112 BindingDB Entry DOI: 10.7270/Q2D21XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50048086 (CHEMBL3311515) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry | Bioorg Med Chem Lett 24: 3298-301 (2014) Article DOI: 10.1016/j.bmcl.2014.06.001 BindingDB Entry DOI: 10.7270/Q2T43VRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 224 total ) | Next | Last >> |