

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activin receptor type-1 (Mus musculus) | BDBM50262079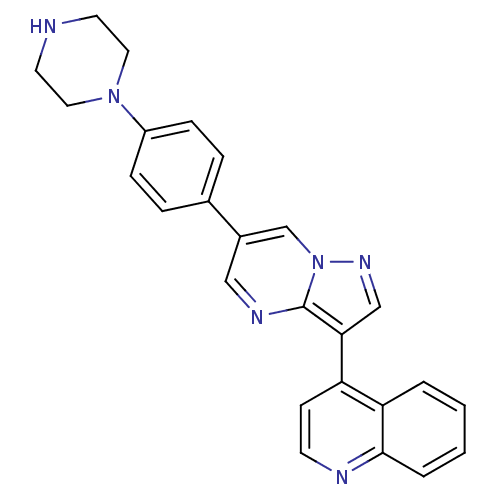 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | ACS Chem Biol 8: 1291-302 (2013) Article DOI: 10.1021/cb300655w BindingDB Entry DOI: 10.7270/Q2ZW1JKW | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603776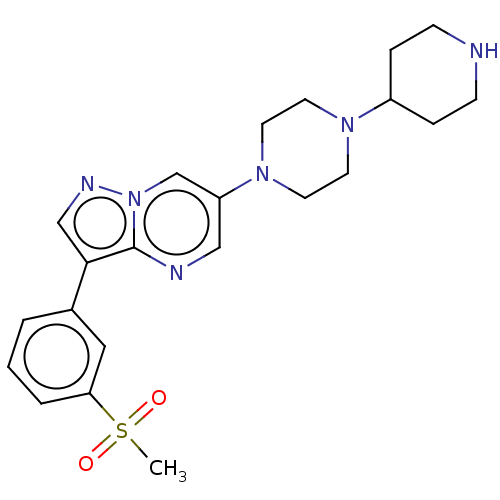 (US11654147, Compound 213) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Mus musculus) | BDBM102619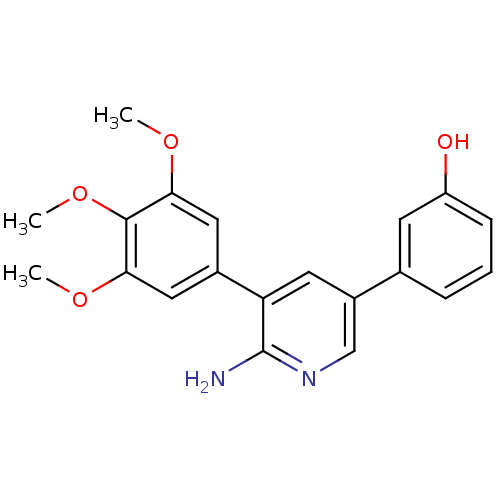 (K02288a | US10688093, Compound 382_0087_0284 | US1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | ACS Chem Biol 8: 1291-302 (2013) Article DOI: 10.1021/cb300655w BindingDB Entry DOI: 10.7270/Q2ZW1JKW | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Mus musculus) | BDBM102618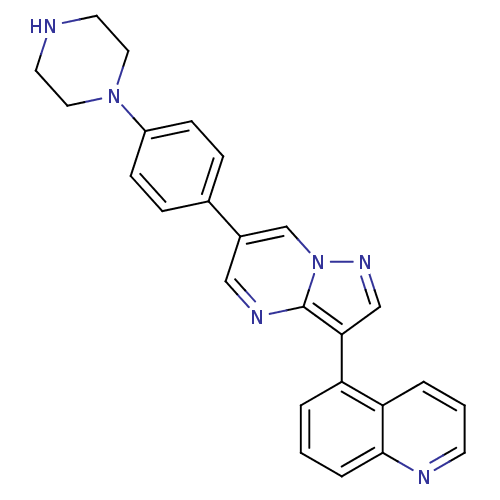 (LDN-212854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | ACS Chem Biol 8: 1291-302 (2013) Article DOI: 10.1021/cb300655w BindingDB Entry DOI: 10.7270/Q2ZW1JKW | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase receptor R3 (Mus musculus) | BDBM50262079 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | ACS Chem Biol 8: 1291-302 (2013) Article DOI: 10.1021/cb300655w BindingDB Entry DOI: 10.7270/Q2ZW1JKW | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603863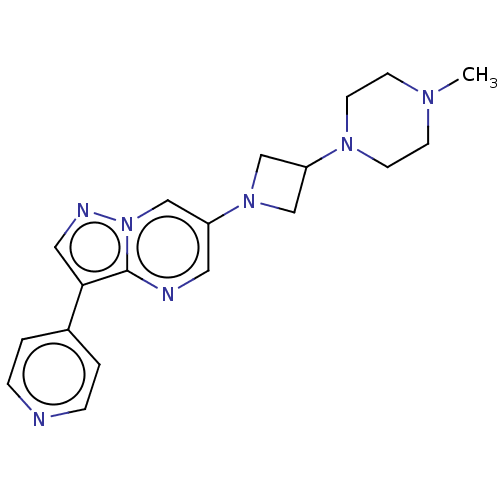 (US11654147, Compound 313 | US11654147, Compound 33...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Mus musculus) | BDBM102618 (LDN-212854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | ACS Chem Biol 8: 1291-302 (2013) Article DOI: 10.1021/cb300655w BindingDB Entry DOI: 10.7270/Q2ZW1JKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603862 (US11654147, Compound 312 | US11654147, Compound 33...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603850 (US11654147, Compound 298) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Mus musculus) | BDBM102619 (K02288a | US10688093, Compound 382_0087_0284 | US1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | ACS Chem Biol 8: 1291-302 (2013) Article DOI: 10.1021/cb300655w BindingDB Entry DOI: 10.7270/Q2ZW1JKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603851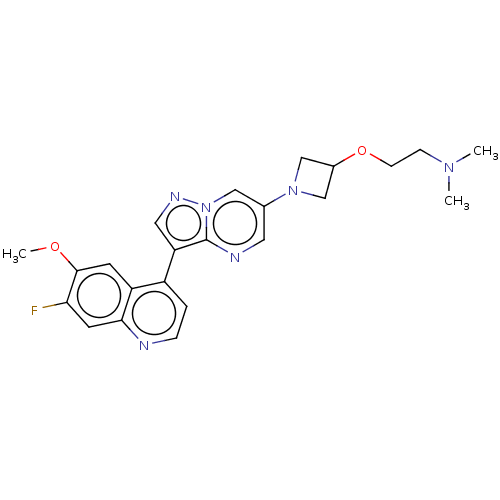 (US11654147, Compound 299 | US11654147, Compound 30...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM111123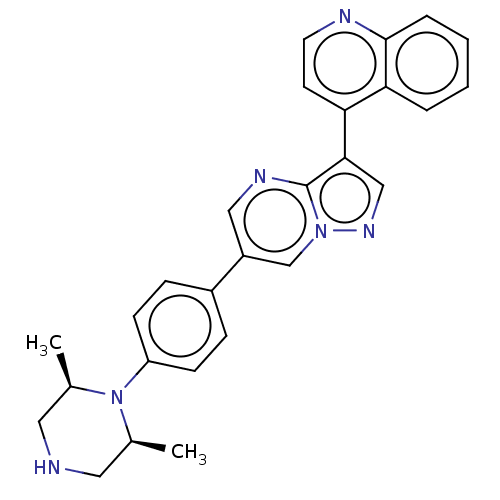 (US10017516, Compound 2 | US9682983, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM143311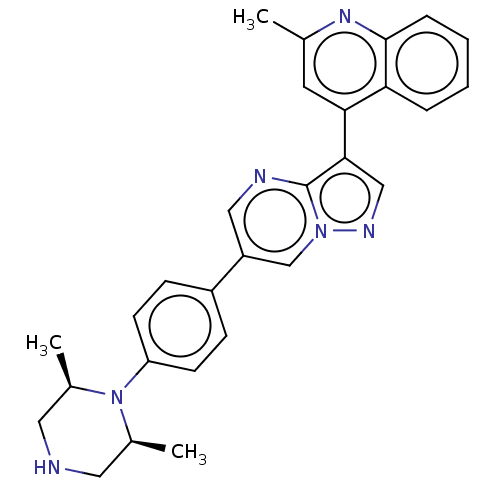 (US10017516, Compound 11 | US9682983, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM143311 (US10017516, Compound 11 | US9682983, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Mus musculus) | BDBM50056508 (CHEMBL3341945) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of BMP6-induced BMP receptor type 1 ALK2 in mouse C2C12 cells after 30 mins by luciferase reporter gene assay | J Med Chem 57: 7900-15 (2014) Article DOI: 10.1021/jm501177w BindingDB Entry DOI: 10.7270/Q2PK0HS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM161920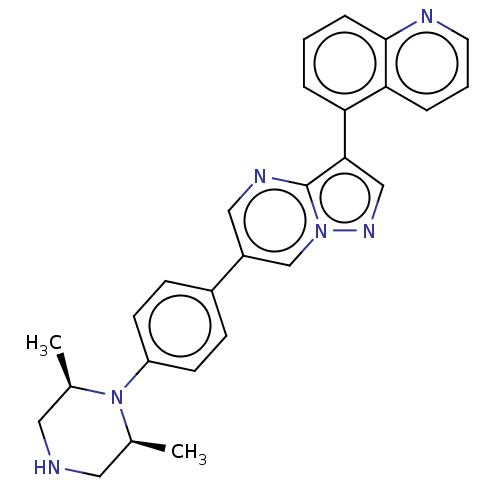 (US10017516, Compound 53 | US9682983, 52 | US968298...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 4 (Homo sapiens (Human)) | BDBM50262079 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of BMP4 (unknown origin)-induced phosphorylation of SMAD 1/5/8 by cytoblot cellular ELISA | Bioorg Med Chem Lett 18: 4388-92 (2008) Article DOI: 10.1016/j.bmcl.2008.06.052 BindingDB Entry DOI: 10.7270/Q2BR8S0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603695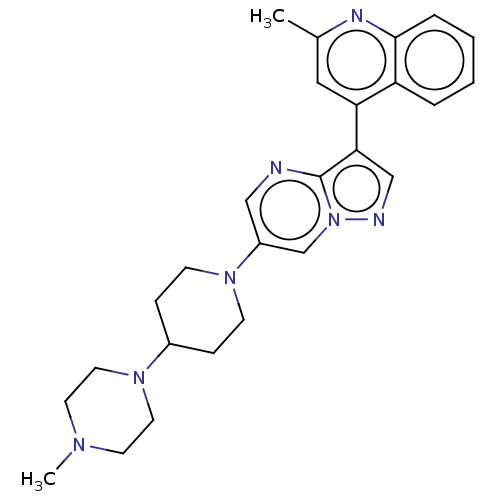 (US11654147, Compound 8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 4 (Homo sapiens (Human)) | BDBM50262178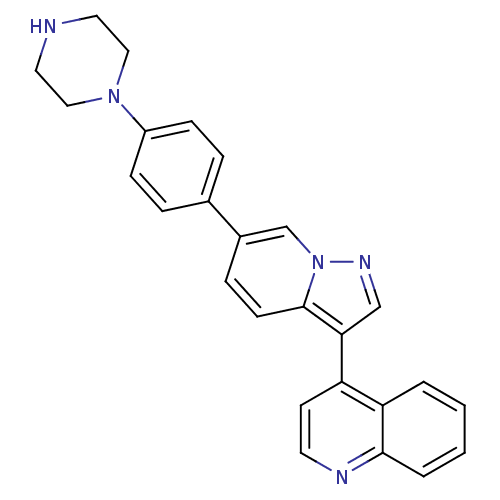 (4-(6-(4-(piperazin-1-yl)phenyl)H-pyrazolo[1,5-a]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of BMP4 (unknown origin)-induced phosphorylation of SMAD 1/5/8 by cytoblot cellular ELISA | Bioorg Med Chem Lett 18: 4388-92 (2008) Article DOI: 10.1016/j.bmcl.2008.06.052 BindingDB Entry DOI: 10.7270/Q2BR8S0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50056506 (CHEMBL3341943 | US10688093, Compound 382_0087_6488...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant human ALK2 kinase after 45 mins by liquid scintillation counting in presence of ATP [gamma-32P] | J Med Chem 57: 7900-15 (2014) Article DOI: 10.1021/jm501177w BindingDB Entry DOI: 10.7270/Q2PK0HS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603784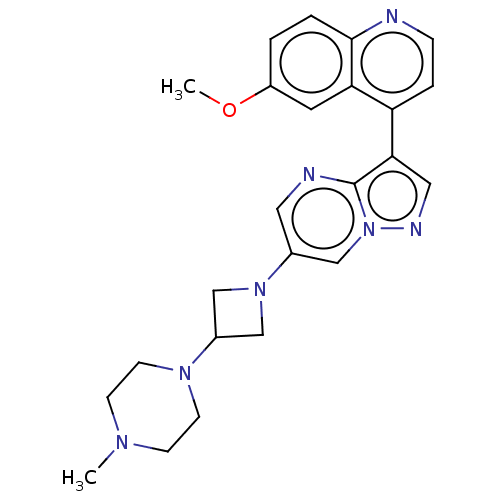 (US11654147, Compound 221) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603845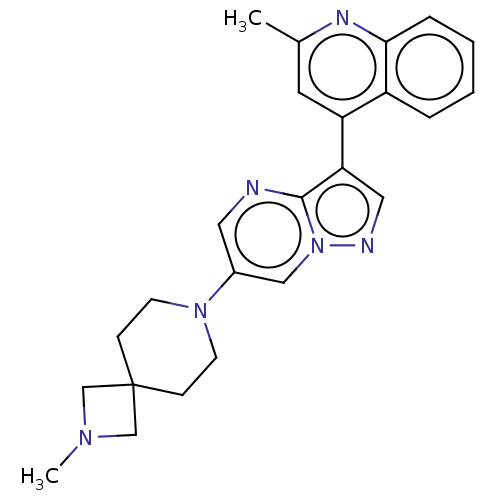 (US11654147, Compound 293) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Mus musculus) | BDBM50056507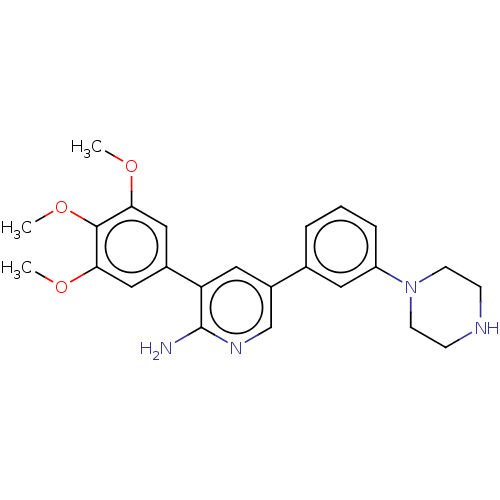 (CHEMBL3341944) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of BMP6-induced BMP receptor type 1 ALK2 in mouse C2C12 cells after 30 mins by luciferase reporter gene assay | J Med Chem 57: 7900-15 (2014) Article DOI: 10.1021/jm501177w BindingDB Entry DOI: 10.7270/Q2PK0HS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603798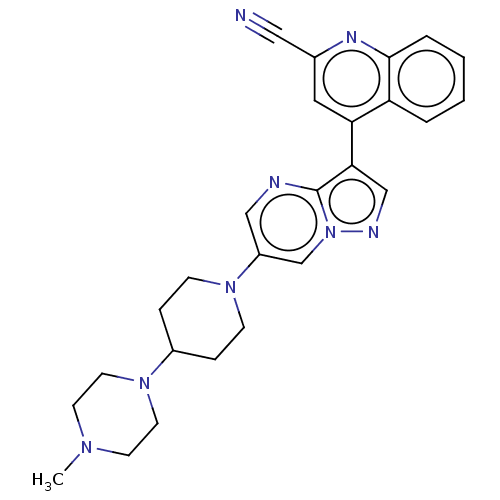 (US11654147, Compound 237 | US11654147, Compound 24...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM161920 (US10017516, Compound 53 | US9682983, 52 | US968298...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603716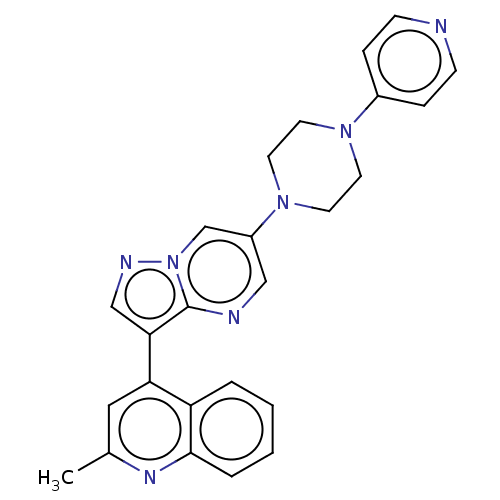 (US11654147, Compound 27) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM143311 (US10017516, Compound 11 | US9682983, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM143311 (US10017516, Compound 11 | US9682983, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603705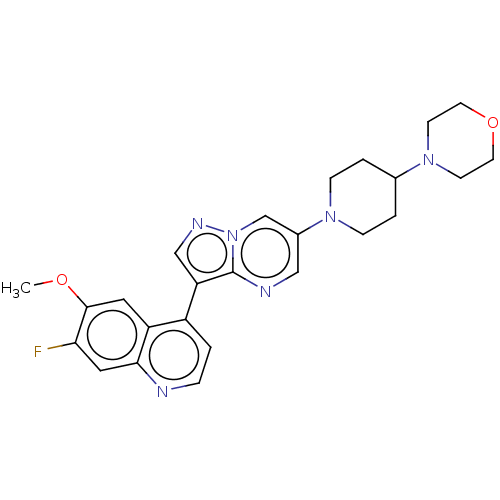 (US11654147, Compound 16) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603709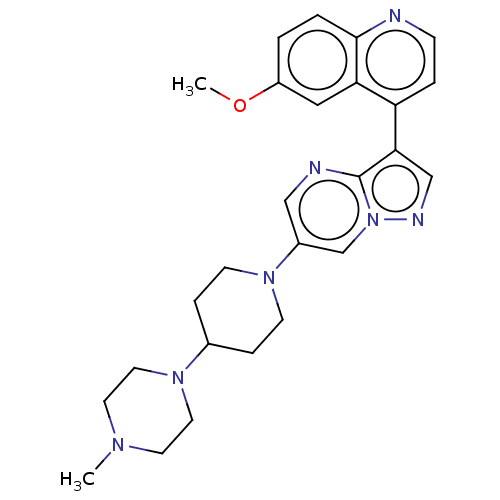 (US11654147, Compound 20) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603757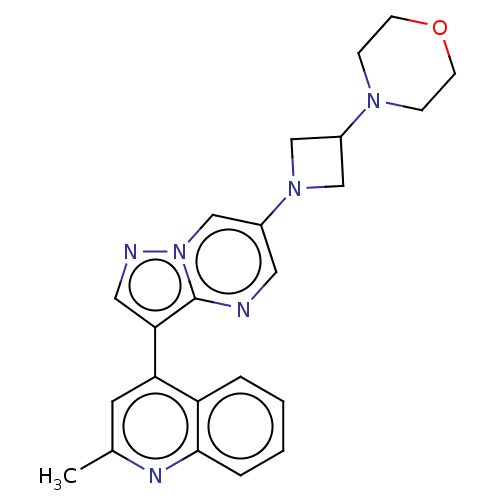 (US11654147, Compound 194 | US11654147, Compound 30...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM161920 (US10017516, Compound 53 | US9682983, 52 | US968298...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603864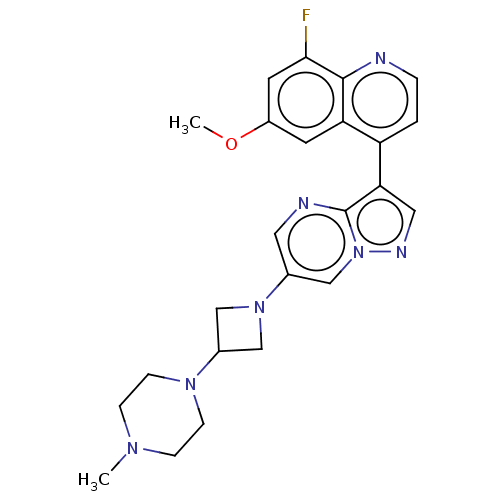 (US11654147, Compound 314) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM161920 (US10017516, Compound 53 | US9682983, 52 | US968298...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM161920 (US10017516, Compound 53 | US9682983, 52 | US968298...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603725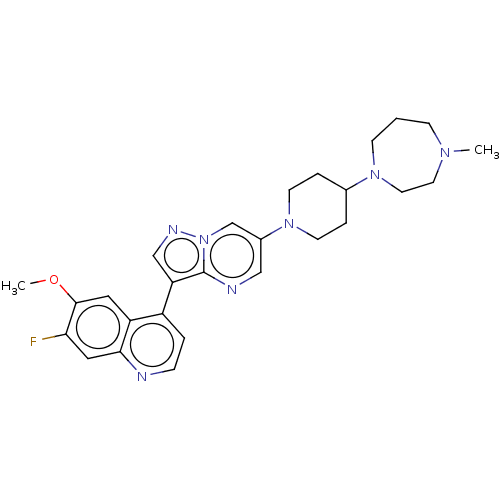 (US11654147, Compound 42) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603735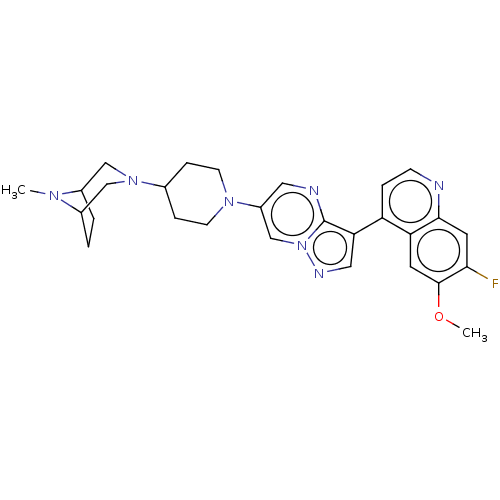 (US11654147, Compound 53) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603737 (US11654147, Compound 55) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM603861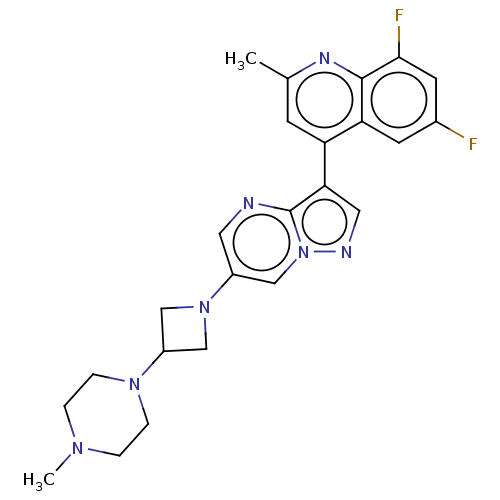 (US11654147, Compound 311 | US11654147, Compound 33...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27085CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Brigham and Women's Hospital, Inc.; Dept. of Health and Human Services, National Institutes of Health US Patent | Assay Description Specifically, compounds were assayed using LANCE« Ultra ULight┐ technology (Perkin Elmer) against human ALK1-6 enzymes (ALK1: Life Technologies, ALK2... | US Patent US9682983 (2017) BindingDB Entry DOI: 10.7270/Q28S4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM143311 (US10017516, Compound 11 | US9682983, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Brigham and Women's Hospital, Inc. US Patent | Assay Description Compounds were assessed in ALK1-6 enzymatic assays. Specifically, compounds were assayed using LANCE« Ultra ULight technology (Perkin Elmer) against ... | US Patent US10017516 (2018) BindingDB Entry DOI: 10.7270/Q20P12CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Mus musculus) | BDBM50262685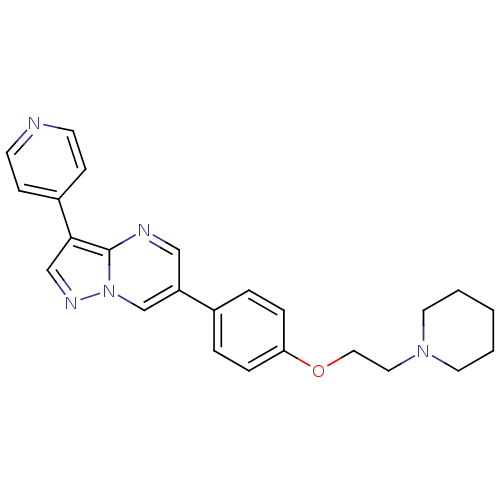 (6-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | ACS Chem Biol 8: 1291-302 (2013) Article DOI: 10.1021/cb300655w BindingDB Entry DOI: 10.7270/Q2ZW1JKW | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1075 total ) | Next | Last >> |