 Found 24357 hits with Last Name = 'zhan' and Initial = 'z'
Found 24357 hits with Last Name = 'zhan' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283
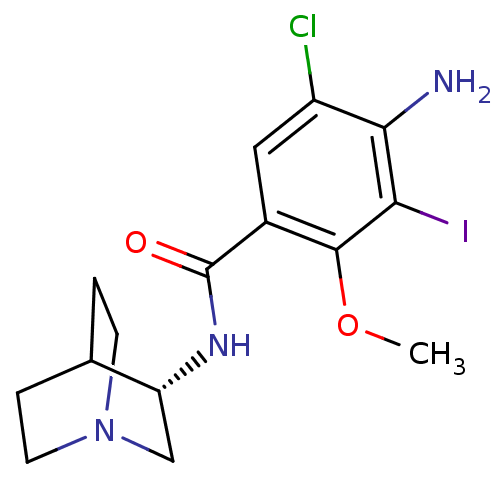
(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50277113
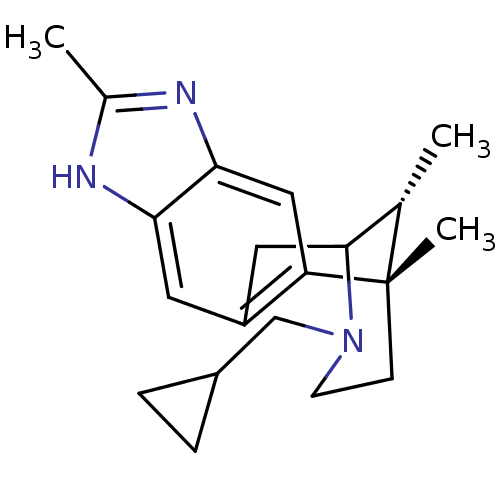
((1S,16R)-13-(cyclopropylmethyl)-1,6,16-trimethyl-5...)Show SMILES C[C@H]1C2Cc3cc4[nH]c(C)nc4cc3[C@@]1(C)CCN2CC1CC1 |r,TLB:12:13:1:18.17.16,19:18:1:4.13.3| Show InChI InChI=1S/C20H27N3/c1-12-19-9-15-8-17-18(22-13(2)21-17)10-16(15)20(12,3)6-7-23(19)11-14-4-5-14/h8,10,12,14,19H,4-7,9,11H2,1-3H3,(H,21,22)/t12-,19?,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 365-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.076
BindingDB Entry DOI: 10.7270/Q2M0458C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283

(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its antagonistic activity against 5-hydroxytryptamine 3 receptor in rat CNS. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334454

(CHEMBL1643895 | Ramosetron | US9045501, Ramosetron)Show SMILES Cn1cc(C(=O)[C@@H]2CCc3nc[nH]c3C2)c2ccccc12 |r| Show InChI InChI=1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50277101
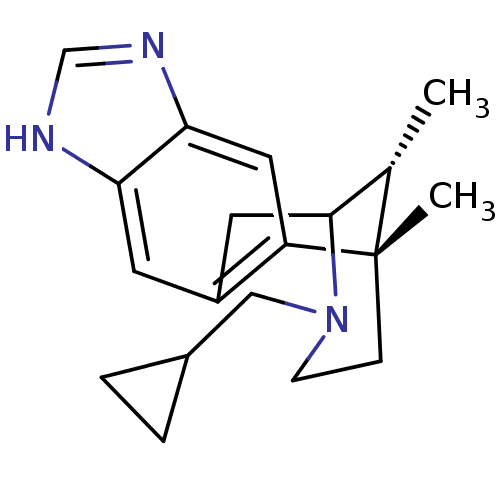
((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...)Show SMILES C[C@H]1C2Cc3cc4[nH]cnc4cc3[C@@]1(C)CCN2CC1CC1 |r,TLB:11:12:1:17.16.15,18:17:1:4.12.3| Show InChI InChI=1S/C19H25N3/c1-12-18-8-14-7-16-17(21-11-20-16)9-15(14)19(12,2)5-6-22(18)10-13-3-4-13/h7,9,11-13,18H,3-6,8,10H2,1-2H3,(H,20,21)/t12-,18?,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 365-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.076
BindingDB Entry DOI: 10.7270/Q2M0458C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50607169
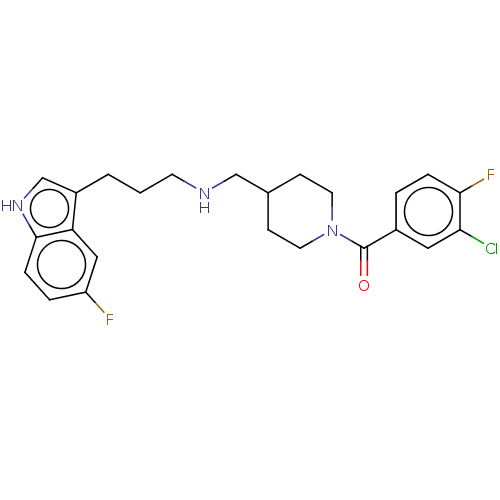
(CHEMBL5220371)Show SMILES Fc1ccc2[nH]cc(CCCNCC3CCN(CC3)C(=O)c3ccc(F)c(Cl)c3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129006
BindingDB Entry DOI: 10.7270/Q2ZC8701 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50349871
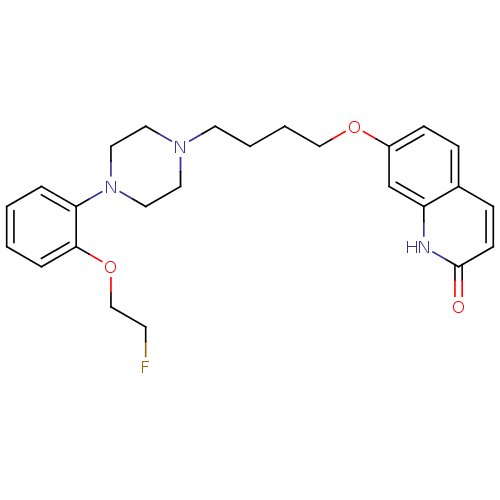
(CHEMBL1813595)Show SMILES FCCOc1ccccc1N1CCN(CCCCOc2ccc3ccc(=O)[nH]c3c2)CC1 Show InChI InChI=1S/C25H30FN3O3/c26-11-18-32-24-6-2-1-5-23(24)29-15-13-28(14-16-29)12-3-4-17-31-21-9-7-20-8-10-25(30)27-22(20)19-21/h1-2,5-10,19H,3-4,11-18H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human recombinant D2L receptor expressed in HEK cells after 60 mins by gamma counter |
Bioorg Med Chem 19: 3502-11 (2011)
Article DOI: 10.1016/j.bmc.2011.04.021
BindingDB Entry DOI: 10.7270/Q25X2990 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50001023
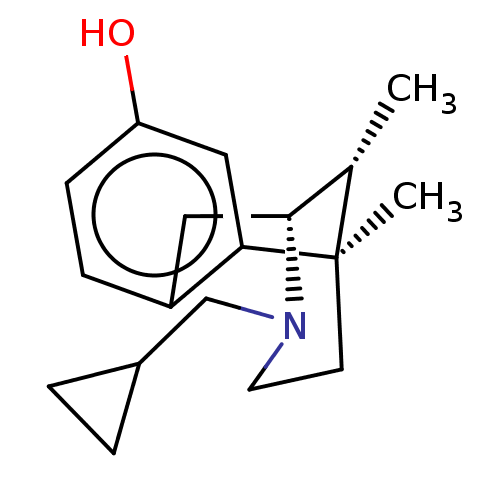
((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CC1CC1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C18H25NO/c1-12-17-9-14-5-6-15(20)10-16(14)18(12,2)7-8-19(17)11-13-3-4-13/h5-6,10,12-13,17,20H,3-4,7-9,11H2,1-2H3/t12-,17+,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 365-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.076
BindingDB Entry DOI: 10.7270/Q2M0458C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283

(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor in whole rat brain using [125I]-DAIZAC as the radioligand. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416
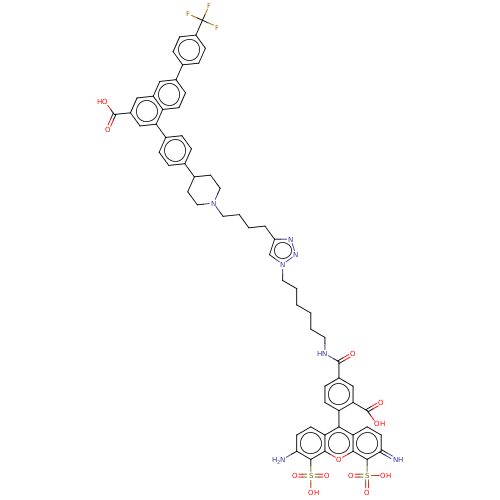
(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14 receptor expressed in CHO cells by fluorescence assay |
Eur J Med Chem 175: 34-39 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.068
BindingDB Entry DOI: 10.7270/Q29C71R4 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600748
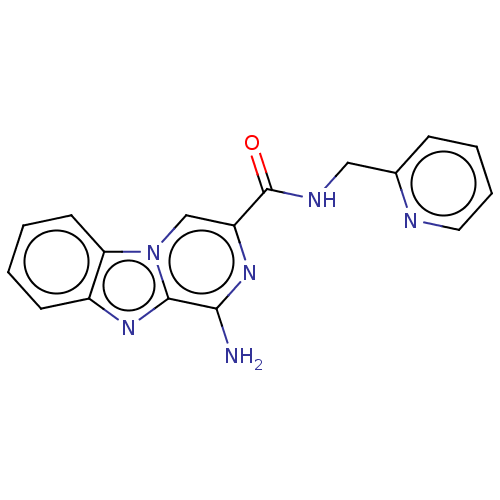
(CHEMBL5176737) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50607170
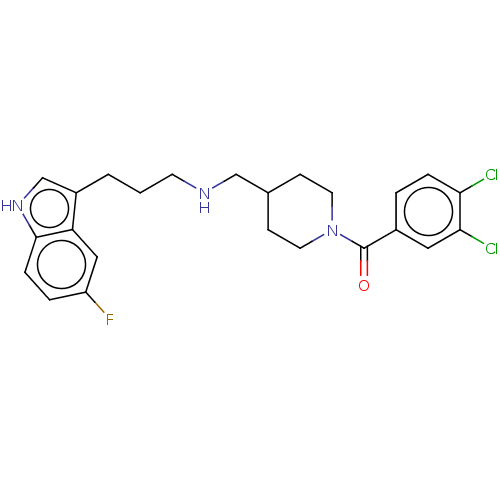
(CHEMBL5220872)Show SMILES Fc1ccc2[nH]cc(CCCNCC3CCN(CC3)C(=O)c3ccc(Cl)c(Cl)c3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129006
BindingDB Entry DOI: 10.7270/Q2ZC8701 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352114
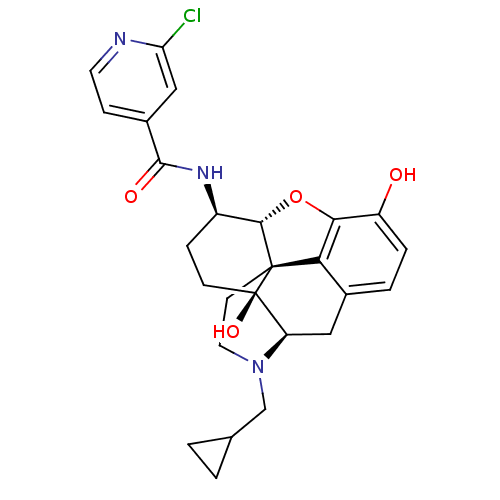
(CHEMBL1824509 | CHEMBL1852788)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Cl)c1 |r| Show InChI InChI=1S/C26H28ClN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571
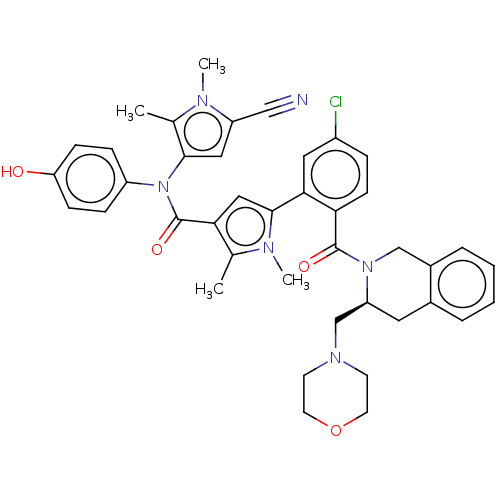
(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50399663
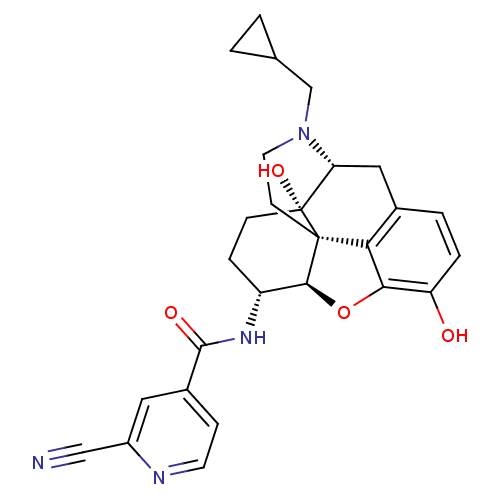
(CHEMBL2177697)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(c1)C#N |r| Show InChI InChI=1S/C27H28N4O4/c28-13-18-11-17(6-9-29-18)25(33)30-19-5-7-27(34)21-12-16-3-4-20(32)23-22(16)26(27,24(19)35-23)8-10-31(21)14-15-1-2-15/h3-4,6,9,11,15,19,21,24,32,34H,1-2,5,7-8,10,12,14H2,(H,30,33)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50349870

(CHEMBL1813594)Show SMILES COc1ccccc1N1CCN(CCCCOc2ccc3ccc(=O)[nH]c3c2)CC1 Show InChI InChI=1S/C24H29N3O3/c1-29-23-7-3-2-6-22(23)27-15-13-26(14-16-27)12-4-5-17-30-20-10-8-19-9-11-24(28)25-21(19)18-20/h2-3,6-11,18H,4-5,12-17H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human recombinant D2L receptor expressed in HEK cells after 60 mins by gamma counter |
Bioorg Med Chem 19: 3502-11 (2011)
Article DOI: 10.1016/j.bmc.2011.04.021
BindingDB Entry DOI: 10.7270/Q25X2990 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50352114

(CHEMBL1824509 | CHEMBL1852788)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Cl)c1 |r| Show InChI InChI=1S/C26H28ClN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001023

((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CC1CC1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C18H25NO/c1-12-17-9-14-5-6-15(20)10-16(14)18(12,2)7-8-19(17)11-13-3-4-13/h5-6,10,12-13,17,20H,3-4,7-9,11H2,1-2H3/t12-,17+,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 365-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.076
BindingDB Entry DOI: 10.7270/Q2M0458C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50352115
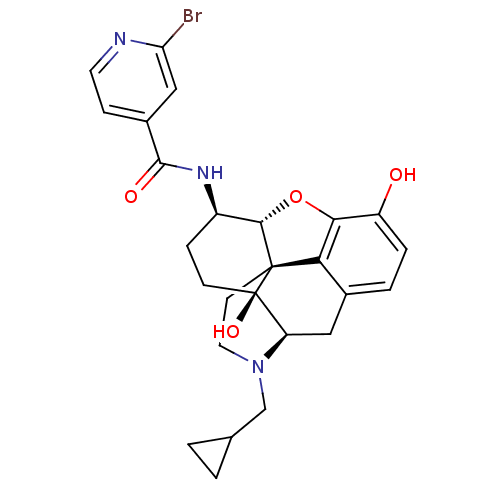
(CHEMBL1824510 | CHEMBL1852385)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccnc(Br)c1 |r| Show InChI InChI=1S/C26H28BrN3O4/c27-20-12-16(6-9-28-20)24(32)29-17-5-7-26(33)19-11-15-3-4-18(31)22-21(15)25(26,23(17)34-22)8-10-30(19)13-14-1-2-14/h3-4,6,9,12,14,17,19,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t17-,19-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50529312

(CHEMBL4444213)Show SMILES Fc1ccc(OCCN2CCC(CC2)c2c[nH]c3ccc(F)cc23)c(c1)-c1ccccc1 Show InChI InChI=1S/C27H26F2N2O/c28-21-6-8-26-24(17-21)25(18-30-26)20-10-12-31(13-11-20)14-15-32-27-9-7-22(29)16-23(27)19-4-2-1-3-5-19/h1-9,16-18,20,30H,10-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129006
BindingDB Entry DOI: 10.7270/Q2ZC8701 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014549
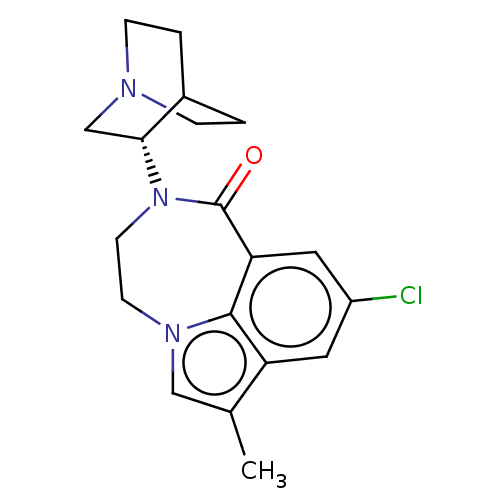
(CHEMBL3261480 | US9045501, 4)Show SMILES Cc1cn2CCN([C@@H]3CN4CCC3CC4)C(=O)c3cc(Cl)cc1c23 |r,wD:7.6,(8.92,-16.19,;8.45,-14.73,;9.36,-13.48,;8.42,-12.13,;8.87,-10.66,;7.99,-9.38,;6.46,-9.26,;5.89,-7.83,;4.36,-7.61,;3.8,-6.17,;4.77,-4.97,;6.29,-5.2,;6.84,-6.63,;5.89,-5.6,;5.13,-6.93,;5.4,-10.41,;3.93,-9.96,;5.64,-11.94,;4.31,-12.71,;4.31,-14.25,;2.98,-15.02,;5.64,-15.02,;6.97,-14.25,;6.98,-12.7,)| Show InChI InChI=1S/C19H22ClN3O/c1-12-10-22-6-7-23(17-11-21-4-2-13(17)3-5-21)19(24)16-9-14(20)8-15(12)18(16)22/h8-10,13,17H,2-7,11H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50349866
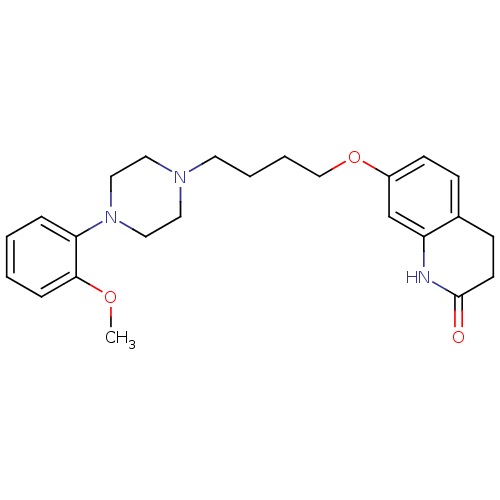
(CHEMBL160296 | CHEMBL1813590 | UNC10108016 | US915...)Show SMILES COc1ccccc1N1CCN(CCCCOc2ccc3CCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H31N3O3/c1-29-23-7-3-2-6-22(23)27-15-13-26(14-16-27)12-4-5-17-30-20-10-8-19-9-11-24(28)25-21(19)18-20/h2-3,6-8,10,18H,4-5,9,11-17H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human recombinant D2L receptor expressed in HEK cells after 60 mins by gamma counter |
Bioorg Med Chem 19: 3502-11 (2011)
Article DOI: 10.1016/j.bmc.2011.04.021
BindingDB Entry DOI: 10.7270/Q25X2990 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50277118
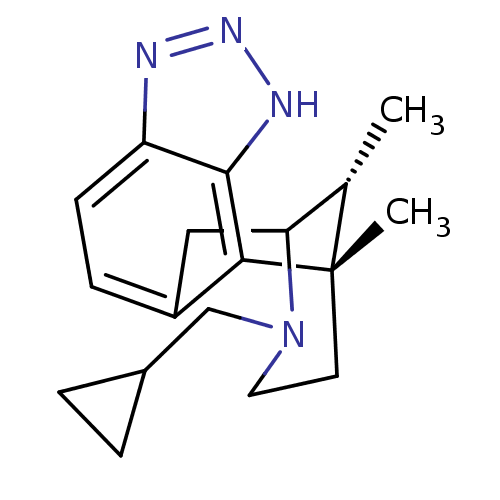
((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-4,5,...)Show SMILES C[C@H]1C2Cc3ccc4nn[nH]c4c3[C@@]1(C)CCN2CC1CC1 |r,TLB:11:12:1:17.16.15,18:17:1:4.12.3| Show InChI InChI=1S/C18H24N4/c1-11-15-9-13-5-6-14-17(20-21-19-14)16(13)18(11,2)7-8-22(15)10-12-3-4-12/h5-6,11-12,15H,3-4,7-10H2,1-2H3,(H,19,20,21)/t11-,15?,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 365-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.076
BindingDB Entry DOI: 10.7270/Q2M0458C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50562972

(CHEMBL4799997)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CCN(C1=O)c1ccc(F)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CAH7 expressed in Escherichia coli BL21 (DE3) by stopped flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02077
BindingDB Entry DOI: 10.7270/Q2C25158 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50349867
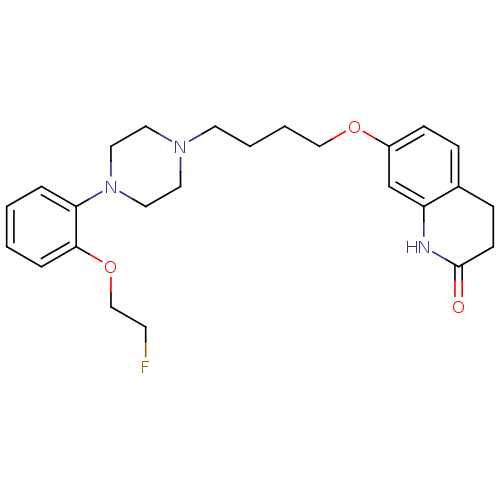
(CHEMBL1813591)Show SMILES FCCOc1ccccc1N1CCN(CCCCOc2ccc3CCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C25H32FN3O3/c26-11-18-32-24-6-2-1-5-23(24)29-15-13-28(14-16-29)12-3-4-17-31-21-9-7-20-8-10-25(30)27-22(20)19-21/h1-2,5-7,9,19H,3-4,8,10-18H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [125I]ABN from human recombinant D2L receptor expressed in HEK cells after 60 mins by gamma counter |
Bioorg Med Chem 19: 3502-11 (2011)
Article DOI: 10.1016/j.bmc.2011.04.021
BindingDB Entry DOI: 10.7270/Q25X2990 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571

(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571

(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594944
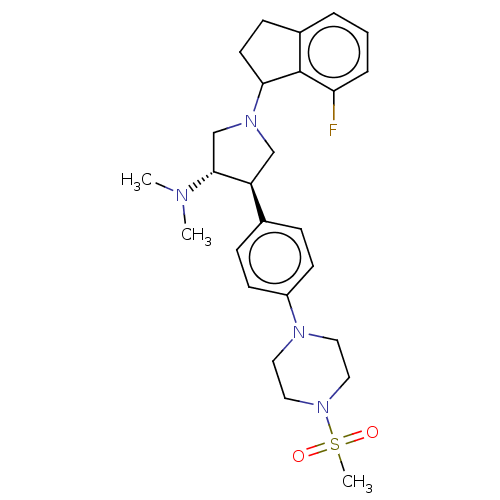
(CHEMBL5181703)Show SMILES CN(C)[C@@H]1CN(C[C@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50099679
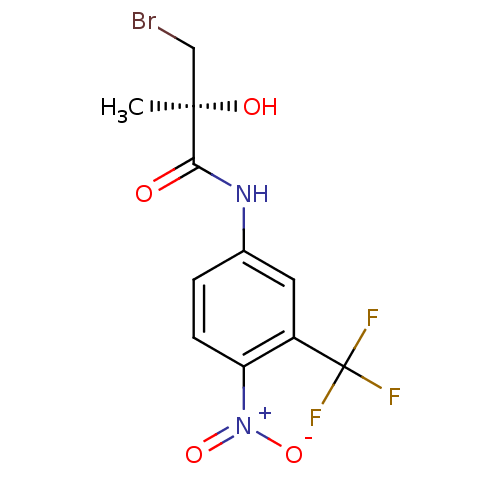
(3-Bromo-2-hydroxy-2-methyl-N-(4-nitro-3-trifluorom...)Show SMILES C[C@@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114119
BindingDB Entry DOI: 10.7270/Q2P55SHP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600748

(CHEMBL5176737) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50277101

((1S,16R)-13-(cyclopropylmethyl)-1,16-dimethyl-5,7,...)Show SMILES C[C@H]1C2Cc3cc4[nH]cnc4cc3[C@@]1(C)CCN2CC1CC1 |r,TLB:11:12:1:17.16.15,18:17:1:4.12.3| Show InChI InChI=1S/C19H25N3/c1-12-18-8-14-7-16-17(21-11-20-16)9-15(14)19(12,2)5-6-22(18)10-13-3-4-13/h7,9,11-13,18H,3-6,8,10H2,1-2H3,(H,20,21)/t12-,18?,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 365-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.076
BindingDB Entry DOI: 10.7270/Q2M0458C |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50590323
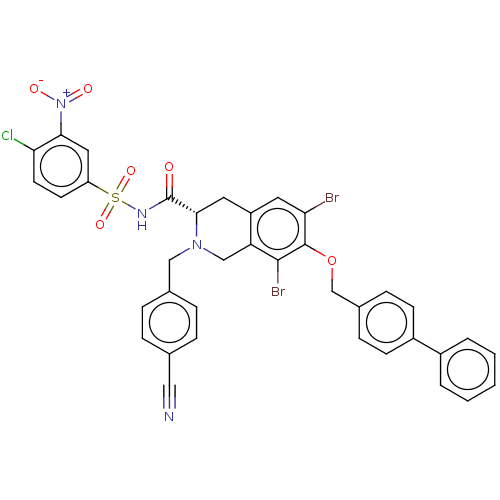
(CHEMBL5173522)Show SMILES [O-][N+](=O)c1cc(ccc1Cl)S(=O)(=O)NC(=O)[C@@H]1Cc2cc(Br)c(OCc3ccc(cc3)-c3ccccc3)c(Br)c2CN1Cc1ccc(cc1)C#N |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588206

(CHEMBL5183968)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6][Si;v4]([#6])([#6])c1cc(-[#8])c-2c(-[#8]C([#6])([#6])c3ccc(-[#6]-[#8])cc-23)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352116
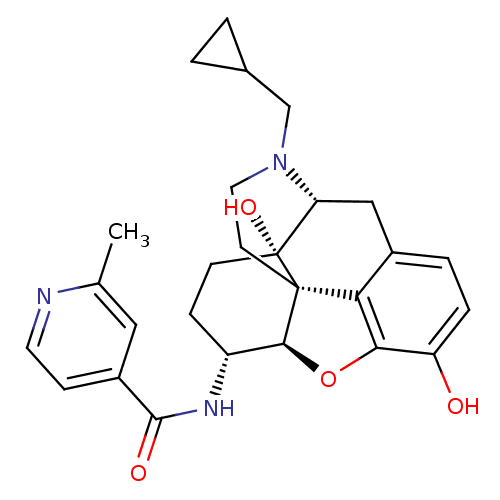
(CHEMBL1824512 | CHEMBL1852558)Show SMILES Cc1cc(ccn1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C27H31N3O4/c1-15-12-18(7-10-28-15)25(32)29-19-6-8-27(33)21-13-17-4-5-20(31)23-22(17)26(27,24(19)34-23)9-11-30(21)14-16-2-3-16/h4-5,7,10,12,16,19,21,24,31,33H,2-3,6,8-9,11,13-14H2,1H3,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50588208
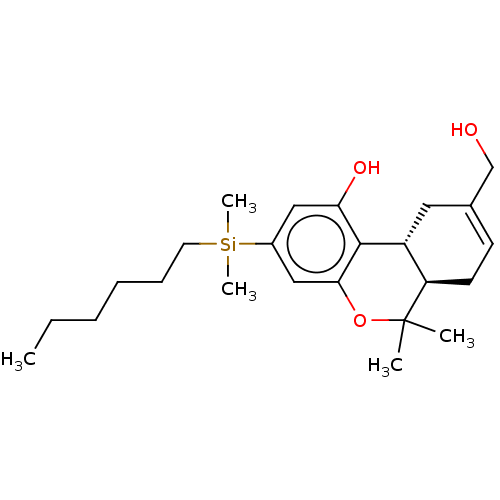
(CHEMBL5187099)Show SMILES [H][C@@]12[#6]-[#6](-[#6]-[#8])=[#6]-[#6][C@@]1([H])C([#6])([#6])[#8]-c1cc(cc(-[#8])c21)[Si;v4]([#6])([#6])[#6]-[#6]-[#6]-[#6]-[#6]-[#6] |r,c:5| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50399661
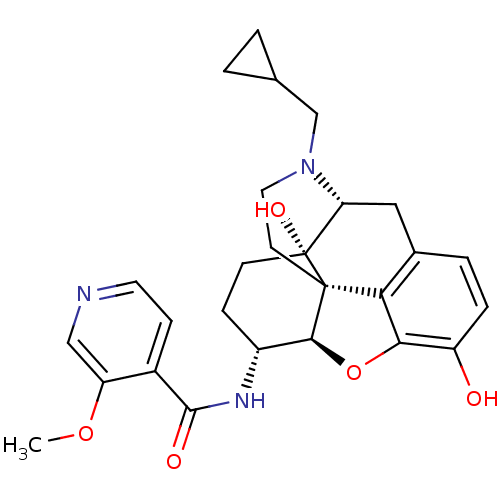
(CHEMBL2178339)Show SMILES COc1cnccc1C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C27H31N3O5/c1-34-20-13-28-10-7-17(20)25(32)29-18-6-8-27(33)21-12-16-4-5-19(31)23-22(16)26(27,24(18)35-23)9-11-30(21)14-15-2-3-15/h4-5,7,10,13,15,18,21,24,31,33H,2-3,6,8-9,11-12,14H2,1H3,(H,29,32)/t18-,21-,24+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352118
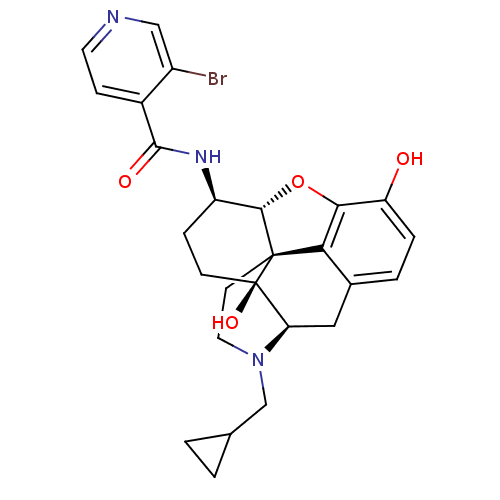
(CHEMBL1824513 | CHEMBL1852602)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccncc1Br |r| Show InChI InChI=1S/C26H28BrN3O4/c27-17-12-28-9-6-16(17)24(32)29-18-5-7-26(33)20-11-15-3-4-19(31)22-21(15)25(26,23(18)34-22)8-10-30(20)13-14-1-2-14/h3-4,6,9,12,14,18,20,23,31,33H,1-2,5,7-8,10-11,13H2,(H,29,32)/t18-,20-,23+,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50590323

(CHEMBL5173522)Show SMILES [O-][N+](=O)c1cc(ccc1Cl)S(=O)(=O)NC(=O)[C@@H]1Cc2cc(Br)c(OCc3ccc(cc3)-c3ccccc3)c(Br)c2CN1Cc1ccc(cc1)C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50399662
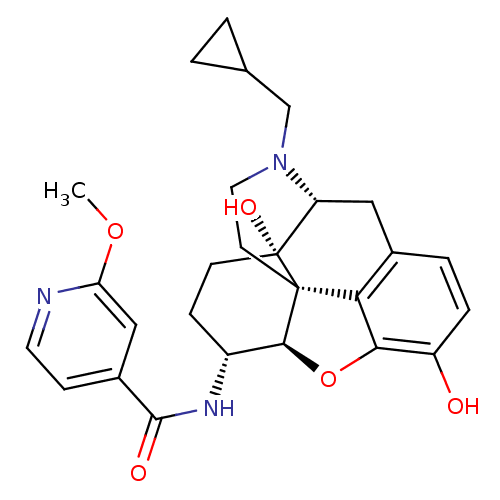
(CHEMBL2178338)Show SMILES COc1cc(ccn1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45 |r| Show InChI InChI=1S/C27H31N3O5/c1-34-21-13-17(7-10-28-21)25(32)29-18-6-8-27(33)20-12-16-4-5-19(31)23-22(16)26(27,24(18)35-23)9-11-30(20)14-15-2-3-15/h4-5,7,10,13,15,18,20,24,31,33H,2-3,6,8-9,11-12,14H2,1H3,(H,29,32)/t18-,20-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50066009
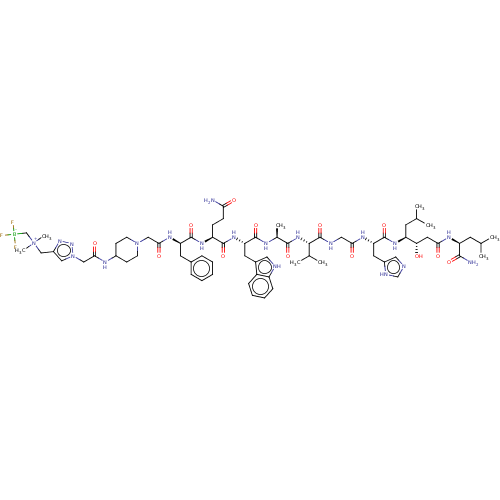
(CHEMBL3401466)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CN1CCC(CC1)NC(=O)Cn1cc(C[N+](C)(C)C[B-](F)(F)F)nn1)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C70H104BF3N20O13/c1-40(2)25-52(57(95)30-59(97)83-53(64(76)101)26-41(3)4)87-69(106)56(29-47-32-77-39-80-47)84-60(98)33-79-70(107)63(42(5)6)89-65(102)43(7)81-67(104)55(28-45-31-78-50-18-14-13-17-49(45)50)88-66(103)51(19-20-58(75)96)86-68(105)54(27-44-15-11-10-12-16-44)85-61(99)35-92-23-21-46(22-24-92)82-62(100)36-93-34-48(90-91-93)37-94(8,9)38-71(72,73)74/h10-18,31-32,34,39-43,46,51-57,63,78,95H,19-30,33,35-38H2,1-9H3,(H2,75,96)(H2,76,101)(H,77,80)(H,79,107)(H,81,104)(H,82,100)(H,83,97)(H,84,98)(H,85,99)(H,86,105)(H,87,106)(H,88,103)(H,89,102)/t43-,51-,52-,53-,54+,55-,56-,57-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr4]bombesin from GRPR (unknown origin) expressed in human PC3 cells after 45 mins by gamma counting analysis |
Bioorg Med Chem 23: 1500-6 (2015)
Article DOI: 10.1016/j.bmc.2015.02.009
BindingDB Entry DOI: 10.7270/Q27H1M8C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775
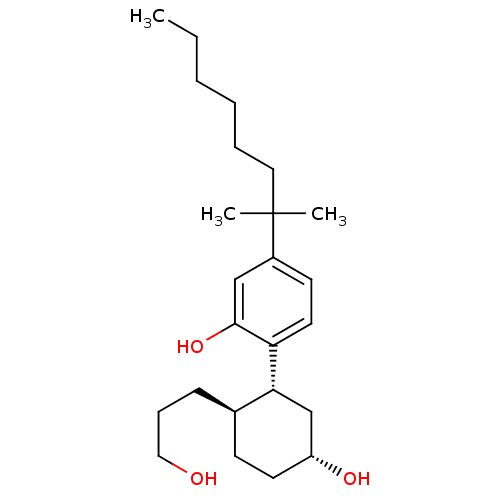
(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113878
BindingDB Entry DOI: 10.7270/Q2PK0M3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014558
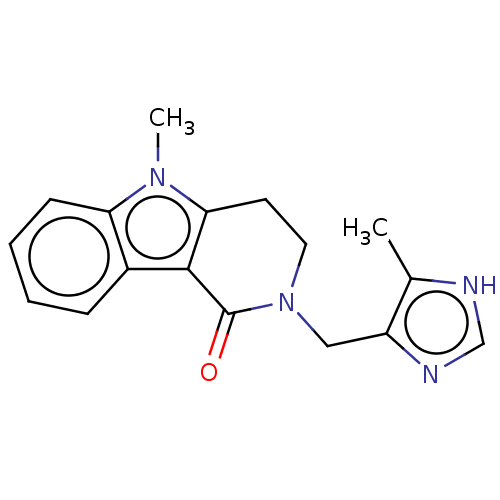
(ALOSETRON | CHEBI:253342 | Lotronex | US9045501, A...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014552
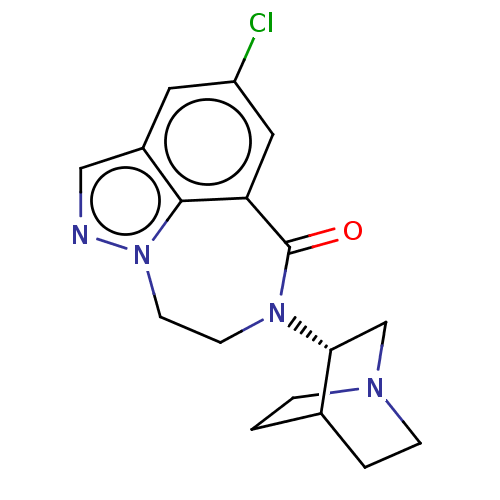
(CHEMBL3261483 | US9045501, 8)Show SMILES Clc1cc2C(=O)N(CCn3ncc(c1)c23)[C@@H]1CN2CCC1CC2 |r,wD:15.17,(22.97,-13.82,;24.31,-13.05,;24.31,-11.51,;25.64,-10.74,;25.4,-9.21,;23.93,-8.77,;26.45,-8.07,;27.99,-8.19,;28.86,-9.46,;28.42,-10.93,;29.36,-12.28,;28.45,-13.53,;26.97,-13.05,;25.64,-13.82,;26.98,-11.51,;25.89,-6.64,;24.36,-6.41,;23.8,-4.97,;24.76,-3.77,;26.29,-4,;26.84,-5.43,;25.89,-4.4,;25.13,-5.74,)| Show InChI InChI=1S/C17H19ClN4O/c18-13-7-12-9-19-22-6-5-21(17(23)14(8-13)16(12)22)15-10-20-3-1-11(15)2-4-20/h7-9,11,15H,1-6,10H2/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM93624
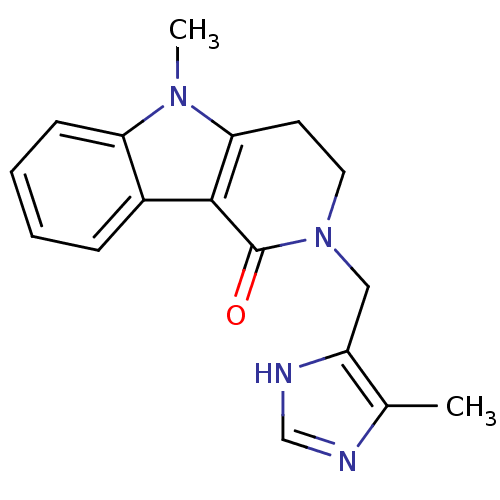
(5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50277117
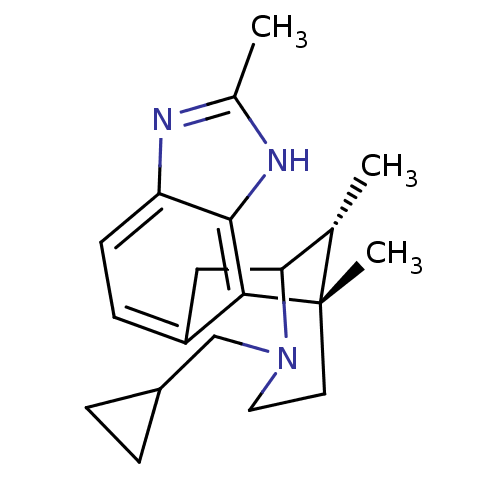
((1S,16R)-13-(cyclopropylmethyl)-1,5,16-trimethyl-4...)Show SMILES C[C@H]1C2Cc3ccc4nc(C)[nH]c4c3[C@@]1(C)CCN2CC1CC1 |r,TLB:12:13:1:18.17.16,19:18:1:4.13.3| Show InChI InChI=1S/C20H27N3/c1-12-17-10-15-6-7-16-19(22-13(2)21-16)18(15)20(12,3)8-9-23(17)11-14-4-5-14/h6-7,12,14,17H,4-5,8-11H2,1-3H3,(H,21,22)/t12-,17?,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 19: 365-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.076
BindingDB Entry DOI: 10.7270/Q2M0458C |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Burkholderia pseudomallei) | BDBM223192
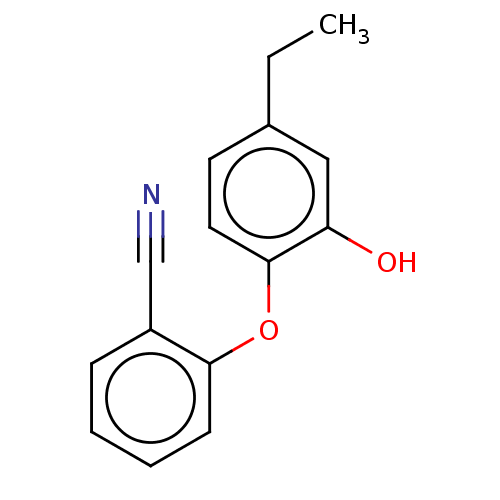
(PT444)Show InChI InChI=1S/C15H13NO2/c1-2-11-7-8-15(13(17)9-11)18-14-6-4-3-5-12(14)10-16/h3-9,17H,2H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | -53.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Slow-onset inhibition kinetics were monitored at 340 nm on a Cary 100 spectrophotometer (Varian) at 25 °C in 30 mM PIPES buffer (pH 8.0) containing 1... |
Biochemistry 56: 1865-1878 (2017)
Article DOI: 10.1021/acs.biochem.6b01048
BindingDB Entry DOI: 10.7270/Q2NP239F |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571

(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50594916
(CHEMBL469759)Show SMILES C[C@@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(I)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114119
BindingDB Entry DOI: 10.7270/Q2P55SHP |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50352121
(CHEMBL1824511 | CHEMBL1852555)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)CCc1ccncc1 |r| Show InChI InChI=1S/C28H33N3O4/c32-21-5-4-19-15-22-28(34)10-7-20(30-23(33)6-3-17-8-12-29-13-9-17)26-27(28,24(19)25(21)35-26)11-14-31(22)16-18-1-2-18/h4-5,8-9,12-13,18,20,22,26,32,34H,1-3,6-7,10-11,14-16H2,(H,30,33)/t20-,22-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr |
J Med Chem 55: 10118-29 (2012)
Article DOI: 10.1021/jm301247n
BindingDB Entry DOI: 10.7270/Q29P32SK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data