 Found 115 hits with Last Name = 'zhao' and Initial = 'jj'
Found 115 hits with Last Name = 'zhao' and Initial = 'jj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-1
(Homo sapiens (Human)) | BDBM50284744

(3-Allyloxycarbonylamino-5-[3-(1H-imidazol-2-yl)-na...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1-c1ncc[nH]1 Show InChI InChI=1S/C22H21N3O6/c1-2-9-30-22(29)25-17(12-20(27)28)18(26)13-31-19-11-15-6-4-3-5-14(15)10-16(19)21-23-7-8-24-21/h2-8,10-11,17H,1,9,12-13H2,(H,23,24)(H,25,29)(H,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284735

(3-Allyloxycarbonylamino-5-(3-carbamoyl-naphthalen-...)Show SMILES NC(=O)c1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C20H20N2O7/c1-2-7-28-20(27)22-15(10-18(24)25)16(23)11-29-17-9-13-6-4-3-5-12(13)8-14(17)19(21)26/h2-6,8-9,15H,1,7,10-11H2,(H2,21,26)(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284734
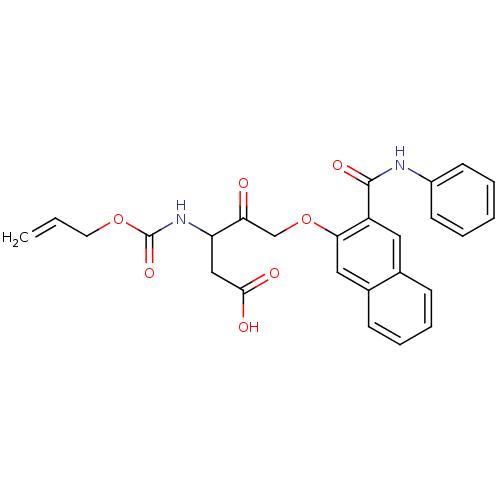
(3-Allyloxycarbonylamino-4-oxo-5-(3-phenylcarbamoyl...)Show SMILES OC(=O)CC(NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1C(=O)Nc1ccccc1 Show InChI InChI=1S/C26H24N2O7/c1-2-12-34-26(33)28-21(15-24(30)31)22(29)16-35-23-14-18-9-7-6-8-17(18)13-20(23)25(32)27-19-10-4-3-5-11-19/h2-11,13-14,21H,1,12,15-16H2,(H,27,32)(H,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284733
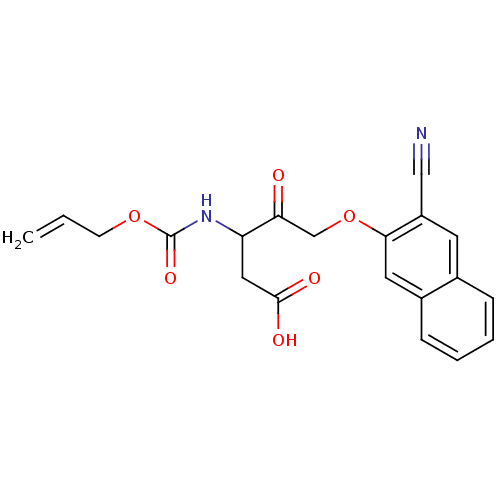
(3-Allyloxycarbonylamino-5-(3-cyano-naphthalen-2-yl...)Show SMILES OC(=O)CC(NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1C#N Show InChI InChI=1S/C20H18N2O6/c1-2-7-27-20(26)22-16(10-19(24)25)17(23)12-28-18-9-14-6-4-3-5-13(14)8-15(18)11-21/h2-6,8-9,16H,1,7,10,12H2,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against ETA receptor in porcine aortic smooth muscle membrane |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284740
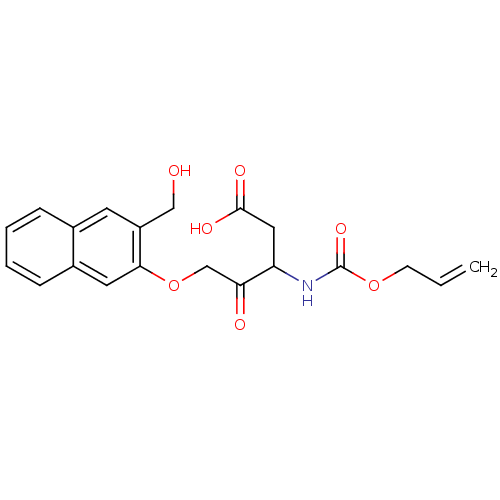
(3-Allyloxycarbonylamino-5-(3-hydroxymethyl-naphtha...)Show SMILES OCc1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C20H21NO7/c1-2-7-27-20(26)21-16(10-19(24)25)17(23)12-28-18-9-14-6-4-3-5-13(14)8-15(18)11-22/h2-6,8-9,16,22H,1,7,10-12H2,(H,21,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50213208
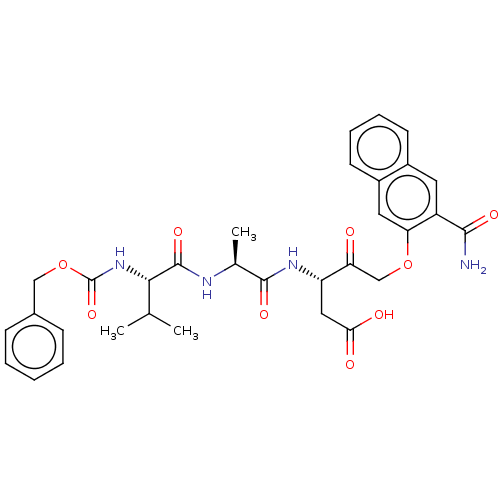
(CHEMBL3143890)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1cc2ccccc2cc1C(N)=O |r| Show InChI InChI=1S/C32H36N4O9/c1-18(2)28(36-32(43)45-16-20-9-5-4-6-10-20)31(42)34-19(3)30(41)35-24(15-27(38)39)25(37)17-44-26-14-22-12-8-7-11-21(22)13-23(26)29(33)40/h4-14,18-19,24,28H,15-17H2,1-3H3,(H2,33,40)(H,34,42)(H,35,41)(H,36,43)(H,38,39)/t19-,24-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against ETA receptor in porcine aortic smooth muscle membrane |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284745

(3-Allyloxycarbonylamino-5-(4-carbamoyl-biphenyl-3-...)Show SMILES NC(=O)c1ccc(cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C)-c1ccccc1 Show InChI InChI=1S/C22H22N2O7/c1-2-10-30-22(29)24-17(12-20(26)27)18(25)13-31-19-11-15(8-9-16(19)21(23)28)14-6-4-3-5-7-14/h2-9,11,17H,1,10,12-13H2,(H2,23,28)(H,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284741
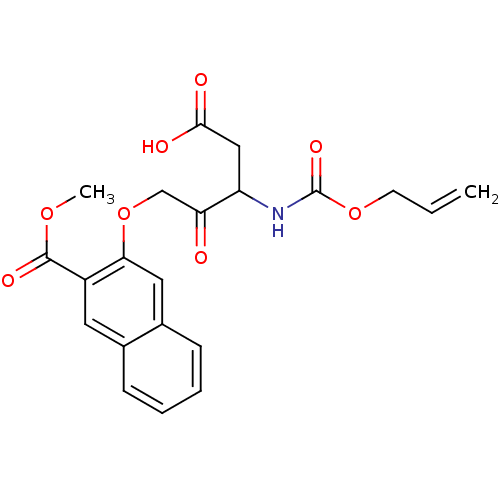
(3-(3-Allyloxycarbonylamino-4-carboxy-2-oxo-butoxy)...)Show SMILES COC(=O)c1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C21H21NO8/c1-3-8-29-21(27)22-16(11-19(24)25)17(23)12-30-18-10-14-7-5-4-6-13(14)9-15(18)20(26)28-2/h3-7,9-10,16H,1,8,11-12H2,2H3,(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284743
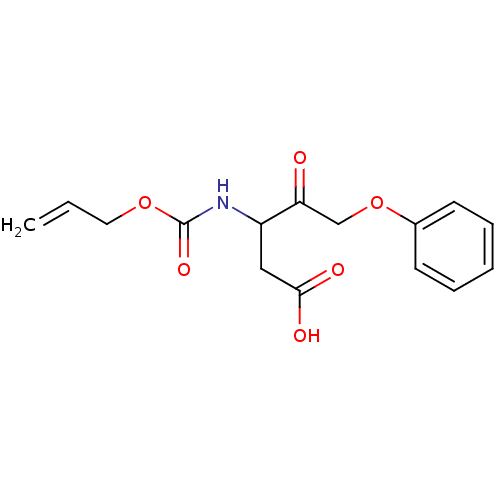
(3-Allyloxycarbonylamino-4-oxo-5-phenoxy-pentanoic ...)Show InChI InChI=1S/C15H17NO6/c1-2-8-21-15(20)16-12(9-14(18)19)13(17)10-22-11-6-4-3-5-7-11/h2-7,12H,1,8-10H2,(H,16,20)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284727
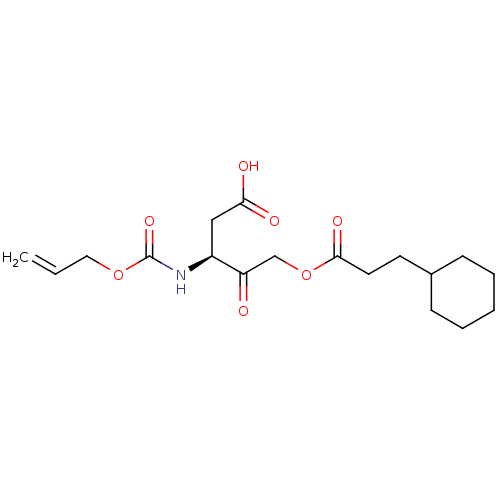
(3-Allyloxycarbonylamino-5-(3-cyclohexyl-propionylo...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COC(=O)CCC1CCCCC1 Show InChI InChI=1S/C18H27NO7/c1-2-10-25-18(24)19-14(11-16(21)22)15(20)12-26-17(23)9-8-13-6-4-3-5-7-13/h2,13-14H,1,3-12H2,(H,19,24)(H,21,22)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284748
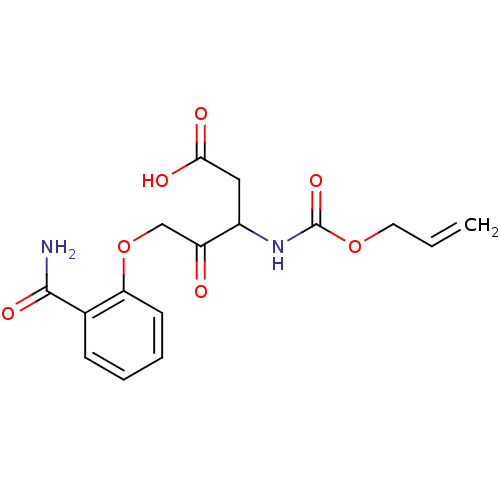
(3-Allyloxycarbonylamino-5-(2-carbamoyl-phenoxy)-4-...)Show InChI InChI=1S/C16H18N2O7/c1-2-7-24-16(23)18-11(8-14(20)21)12(19)9-25-13-6-4-3-5-10(13)15(17)22/h2-6,11H,1,7-9H2,(H2,17,22)(H,18,23)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284749
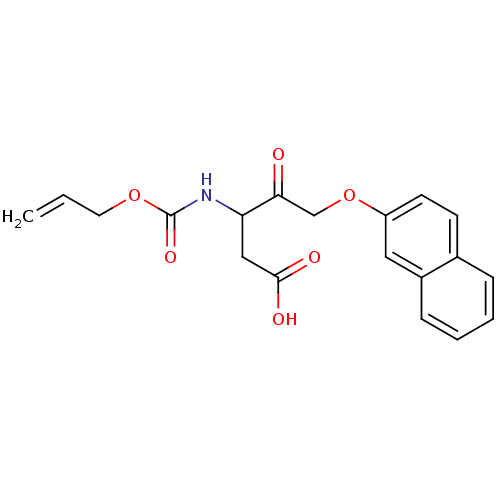
(3-Allyloxycarbonylamino-5-(naphthalen-2-yloxy)-4-o...)Show InChI InChI=1S/C19H19NO6/c1-2-9-25-19(24)20-16(11-18(22)23)17(21)12-26-15-8-7-13-5-3-4-6-14(13)10-15/h2-8,10,16H,1,9,11-12H2,(H,20,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284732
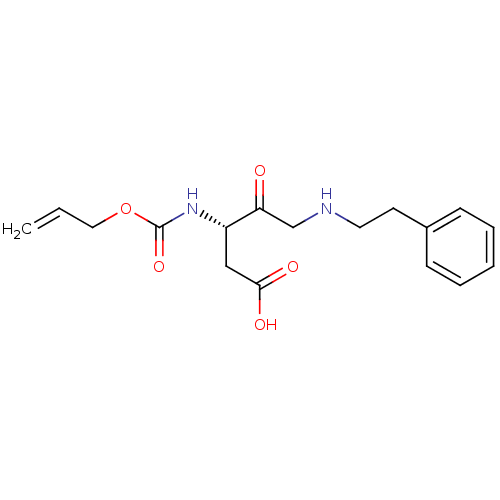
(3-Allyloxycarbonylamino-4-oxo-5-phenethylamino-pen...)Show InChI InChI=1S/C17H22N2O5/c1-2-10-24-17(23)19-14(11-16(21)22)15(20)12-18-9-8-13-6-4-3-5-7-13/h2-7,14,18H,1,8-12H2,(H,19,23)(H,21,22)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284739

(3-[2-(2-Benzyloxycarbonylamino-3-methyl-butyrylami...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 |r| Show InChI InChI=1S/C27H33N3O8/c1-17(2)24(30-27(36)38-15-19-10-6-4-7-11-19)26(35)28-18(3)25(34)29-21(14-23(32)33)22(31)16-37-20-12-8-5-9-13-20/h4-13,17-18,21,24H,14-16H2,1-3H3,(H,28,35)(H,29,34)(H,30,36)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284747
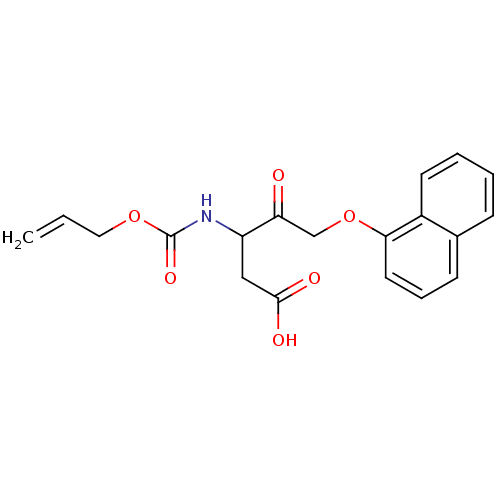
(3-Allyloxycarbonylamino-5-(naphthalen-1-yloxy)-4-o...)Show InChI InChI=1S/C19H19NO6/c1-2-10-25-19(24)20-15(11-18(22)23)16(21)12-26-17-9-5-7-13-6-3-4-8-14(13)17/h2-9,15H,1,10-12H2,(H,20,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284737
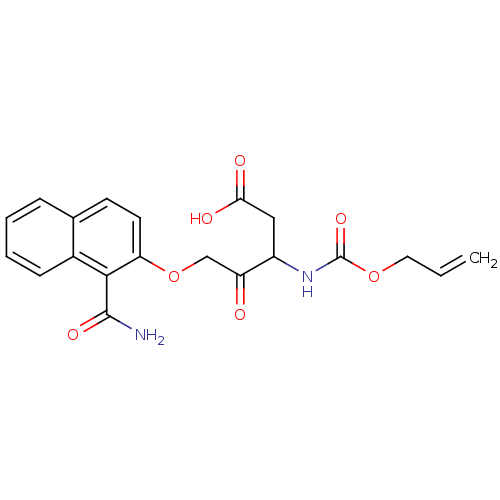
(3-Allyloxycarbonylamino-5-(1-carbamoyl-naphthalen-...)Show SMILES NC(=O)c1c(OCC(=O)C(CC(O)=O)NC(=O)OCC=C)ccc2ccccc12 Show InChI InChI=1S/C20H20N2O7/c1-2-9-28-20(27)22-14(10-17(24)25)15(23)11-29-16-8-7-12-5-3-4-6-13(12)18(16)19(21)26/h2-8,14H,1,9-11H2,(H2,21,26)(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284724
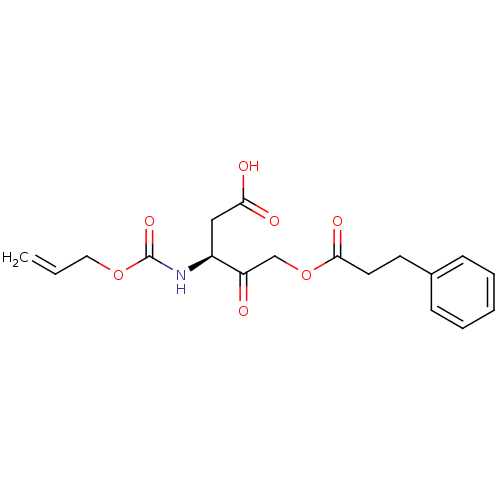
(3-Allyloxycarbonylamino-4-oxo-5-(3-phenyl-propiony...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COC(=O)CCc1ccccc1 Show InChI InChI=1S/C18H21NO7/c1-2-10-25-18(24)19-14(11-16(21)22)15(20)12-26-17(23)9-8-13-6-4-3-5-7-13/h2-7,14H,1,8-12H2,(H,19,24)(H,21,22)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284736
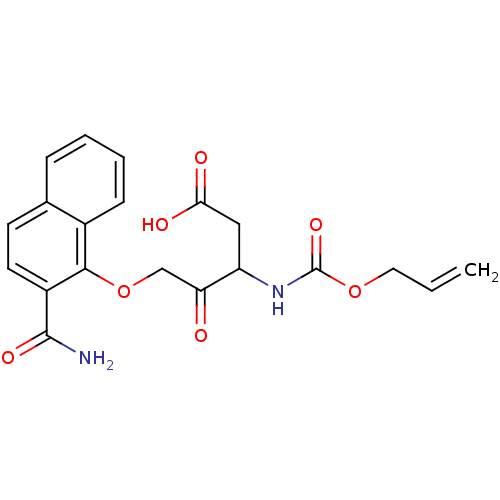
(3-Allyloxycarbonylamino-5-(2-carbamoyl-naphthalen-...)Show SMILES NC(=O)c1ccc2ccccc2c1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C20H20N2O7/c1-2-9-28-20(27)22-15(10-17(24)25)16(23)11-29-18-13-6-4-3-5-12(13)7-8-14(18)19(21)26/h2-8,15H,1,9-11H2,(H2,21,26)(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284722
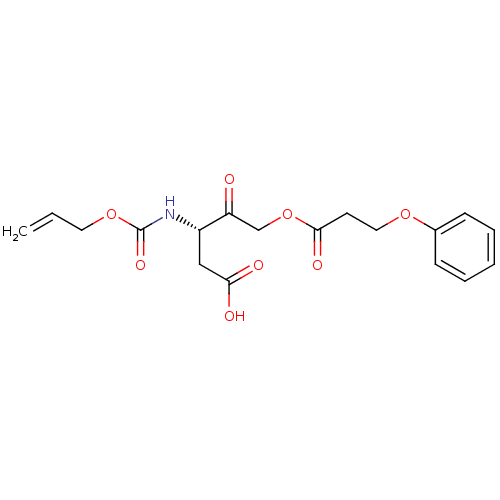
(3-Allyloxycarbonylamino-4-oxo-5-(3-phenoxy-propion...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COC(=O)CCOc1ccccc1 Show InChI InChI=1S/C18H21NO8/c1-2-9-26-18(24)19-14(11-16(21)22)15(20)12-27-17(23)8-10-25-13-6-4-3-5-7-13/h2-7,14H,1,8-12H2,(H,19,24)(H,21,22)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284718

(3-Benzyloxycarbonylamino-4-oxo-5-(3-phenyl-propoxy...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)COCCCc1ccccc1 Show InChI InChI=1S/C22H25NO6/c24-20(16-28-13-7-12-17-8-3-1-4-9-17)19(14-21(25)26)23-22(27)29-15-18-10-5-2-6-11-18/h1-6,8-11,19H,7,12-16H2,(H,23,27)(H,25,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284726
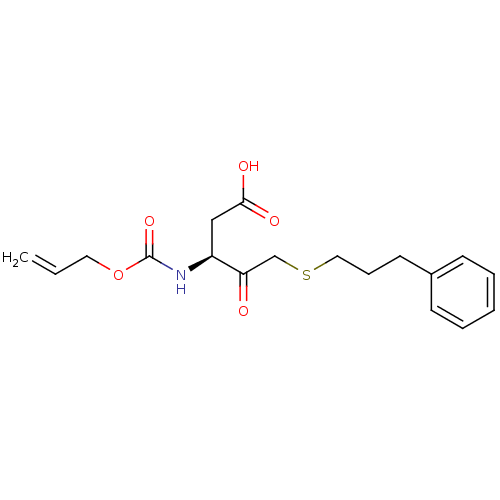
(3-Allyloxycarbonylamino-4-oxo-5-(3-phenyl-propylsu...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)CSCCCc1ccccc1 Show InChI InChI=1S/C18H23NO5S/c1-2-10-24-18(23)19-15(12-17(21)22)16(20)13-25-11-6-9-14-7-4-3-5-8-14/h2-5,7-8,15H,1,6,9-13H2,(H,19,23)(H,21,22)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284738
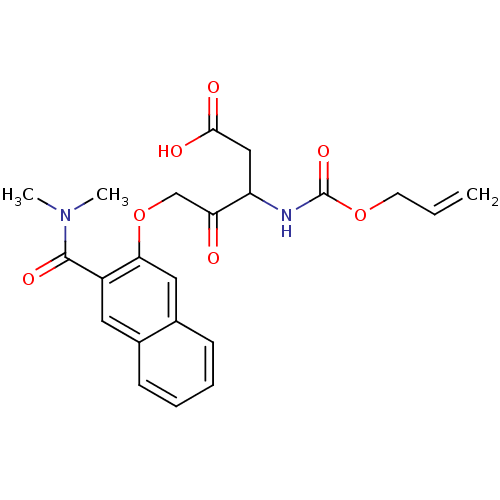
(3-Allyloxycarbonylamino-5-(3-dimethylcarbamoyl-nap...)Show SMILES CN(C)C(=O)c1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C22H24N2O7/c1-4-9-30-22(29)23-17(12-20(26)27)18(25)13-31-19-11-15-8-6-5-7-14(15)10-16(19)21(28)24(2)3/h4-8,10-11,17H,1,9,12-13H2,2-3H3,(H,23,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50281182
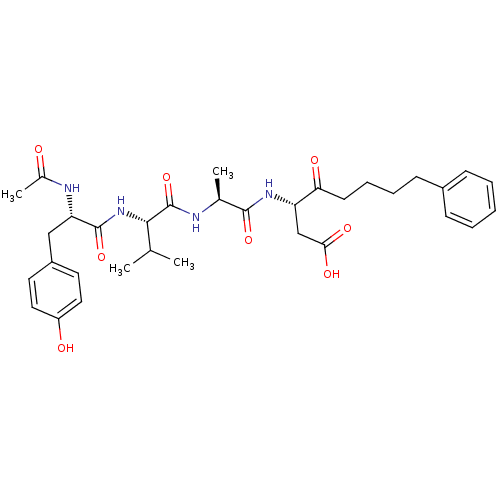
(3-(2-{2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propio...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCc1ccccc1 Show InChI InChI=1S/C33H44N4O8/c1-20(2)30(37-32(44)27(35-22(4)38)18-24-14-16-25(39)17-15-24)33(45)34-21(3)31(43)36-26(19-29(41)42)28(40)13-9-8-12-23-10-6-5-7-11-23/h5-7,10-11,14-17,20-21,26-27,30,39H,8-9,12-13,18-19H2,1-4H3,(H,34,45)(H,35,38)(H,36,43)(H,37,44)(H,41,42)/t21-,26-,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284731
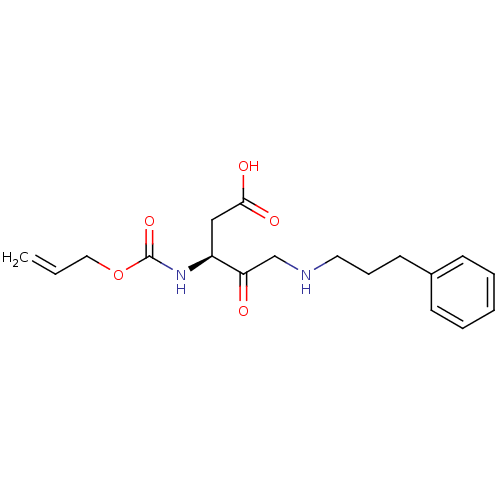
(3-Allyloxycarbonylamino-4-oxo-5-(3-phenyl-propylam...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)CNCCCc1ccccc1 Show InChI InChI=1S/C18H24N2O5/c1-2-11-25-18(24)20-15(12-17(22)23)16(21)13-19-10-6-9-14-7-4-3-5-8-14/h2-5,7-8,15,19H,1,6,9-13H2,(H,20,24)(H,22,23)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284723

(3-Allyloxycarbonylamino-5-(benzyl-phenethyl-amino)...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)CN(CCc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C24H28N2O5/c1-2-15-31-24(30)25-21(16-23(28)29)22(27)18-26(17-20-11-7-4-8-12-20)14-13-19-9-5-3-6-10-19/h2-12,21H,1,13-18H2,(H,25,30)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284729
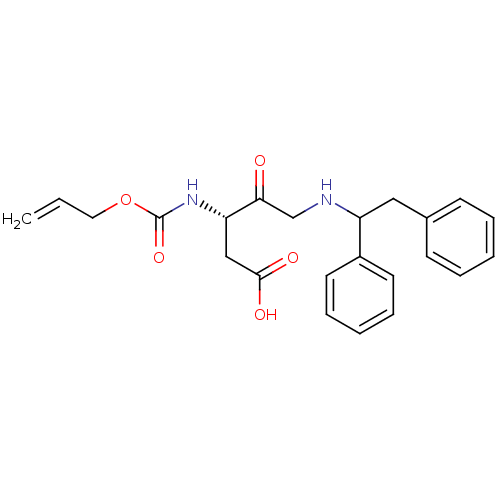
(3-Allyloxycarbonylamino-5-(1,2-diphenyl-ethylamino...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)CNC(Cc1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H26N2O5/c1-2-13-30-23(29)25-20(15-22(27)28)21(26)16-24-19(18-11-7-4-8-12-18)14-17-9-5-3-6-10-17/h2-12,19-20,24H,1,13-16H2,(H,25,29)(H,27,28)/t19?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284725
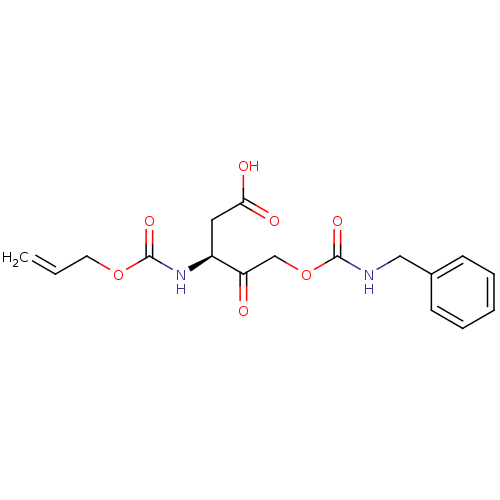
(3-Allyloxycarbonylamino-5-benzylcarbamoyloxy-4-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COC(=O)NCc1ccccc1 Show InChI InChI=1S/C17H20N2O7/c1-2-8-25-17(24)19-13(9-15(21)22)14(20)11-26-16(23)18-10-12-6-4-3-5-7-12/h2-7,13H,1,8-11H2,(H,18,23)(H,19,24)(H,21,22)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284719
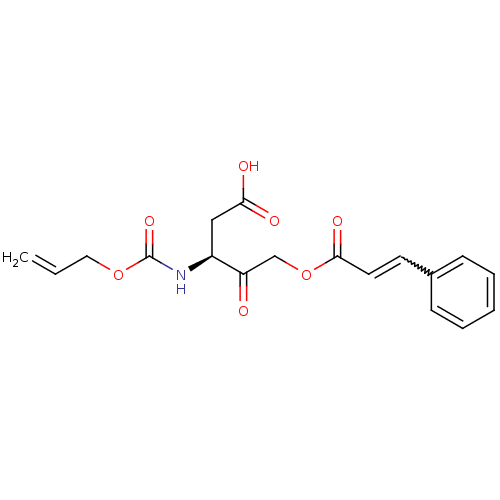
((S)-3-Allyloxycarbonylamino-4-oxo-5-[(E)-(3-phenyl...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COC(=O)C=Cc1ccccc1 |w:19.19| Show InChI InChI=1S/C18H19NO7/c1-2-10-25-18(24)19-14(11-16(21)22)15(20)12-26-17(23)9-8-13-6-4-3-5-7-13/h2-9,14H,1,10-12H2,(H,19,24)(H,21,22)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
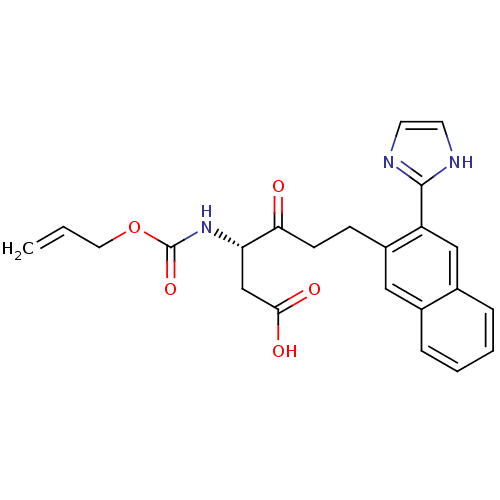
(Homo sapiens (Human)) | BDBM50284746
(3-Allyloxycarbonylamino-6-[3-(1H-imidazol-2-yl)-na...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)CCc1cc2ccccc2cc1-c1ncc[nH]1 Show InChI InChI=1S/C23H23N3O5/c1-2-11-31-23(30)26-19(14-21(28)29)20(27)8-7-17-12-15-5-3-4-6-16(15)13-18(17)22-24-9-10-25-22/h2-6,9-10,12-13,19H,1,7-8,11,14H2,(H,24,25)(H,26,30)(H,28,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
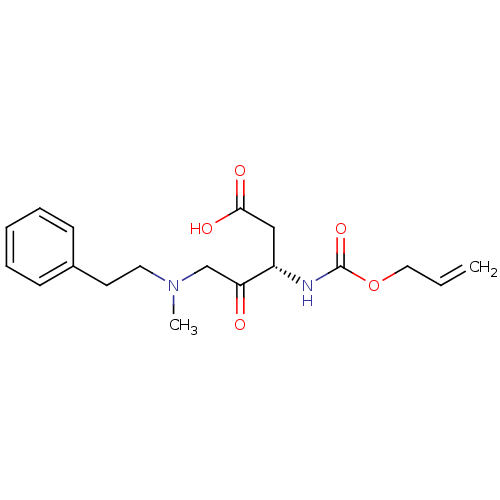
(Homo sapiens (Human)) | BDBM50284728
(3-Allyloxycarbonylamino-5-(methyl-phenethyl-amino)...)Show SMILES CN(CCc1ccccc1)CC(=O)[C@H](CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C18H24N2O5/c1-3-11-25-18(24)19-15(12-17(22)23)16(21)13-20(2)10-9-14-7-5-4-6-8-14/h3-8,15H,1,9-13H2,2H3,(H,19,24)(H,22,23)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284721
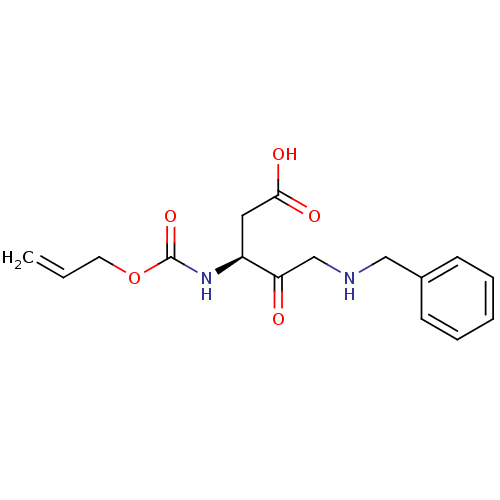
(3-Allyloxycarbonylamino-5-benzylamino-4-oxo-pentan...)Show InChI InChI=1S/C16H20N2O5/c1-2-8-23-16(22)18-13(9-15(20)21)14(19)11-17-10-12-6-4-3-5-7-12/h2-7,13,17H,1,8-11H2,(H,18,22)(H,20,21)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284720
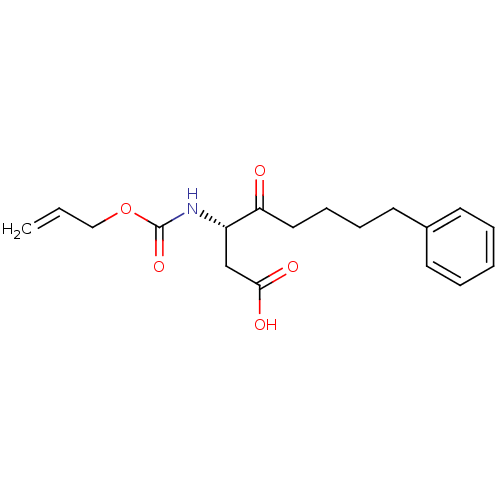
(3-Allyloxycarbonylamino-4-oxo-8-phenyl-octanoic ac...)Show InChI InChI=1S/C18H23NO5/c1-2-12-24-18(23)19-15(13-17(21)22)16(20)11-7-6-10-14-8-4-3-5-9-14/h2-5,8-9,15H,1,6-7,10-13H2,(H,19,23)(H,21,22)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284730
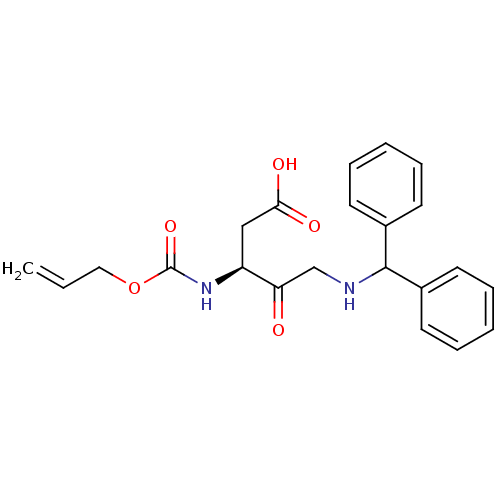
(3-Allyloxycarbonylamino-5-(benzhydryl-amino)-4-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)CNC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H24N2O5/c1-2-13-29-22(28)24-18(14-20(26)27)19(25)15-23-21(16-9-5-3-6-10-16)17-11-7-4-8-12-17/h2-12,18,21,23H,1,13-15H2,(H,24,28)(H,26,27)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes |
Bioorg Med Chem Lett 5: 1405-1408 (1995)
Article DOI: 10.1016/0960-894X(95)00231-H
BindingDB Entry DOI: 10.7270/Q26T0MKT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50193013

(Duvelisib | INK-1147 | INK-1197 | IPI-145)Show SMILES C[C@H](Nc1ncnc2nc[nH]c12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H17ClN6O/c1-13(28-21-19-20(25-11-24-19)26-12-27-21)17-10-14-6-5-9-16(23)18(14)22(30)29(17)15-7-3-2-4-8-15/h2-13H,1H3,(H2,24,25,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50193032
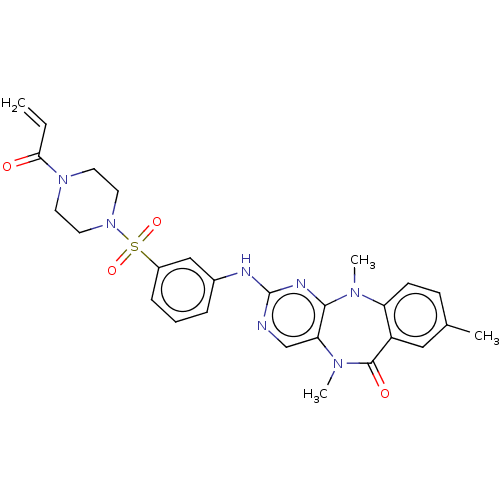
(CHEMBL3979343 | US11155556, No. 2)Show SMILES CN1c2ccc(C)cc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(=O)(=O)N3CCN(CC3)C(=O)C=C)nc12 Show InChI InChI=1S/C27H29N7O4S/c1-5-24(35)33-11-13-34(14-12-33)39(37,38)20-8-6-7-19(16-20)29-27-28-17-23-25(30-27)31(3)22-10-9-18(2)15-21(22)26(36)32(23)4/h5-10,15-17H,1,11-14H2,2-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50193014
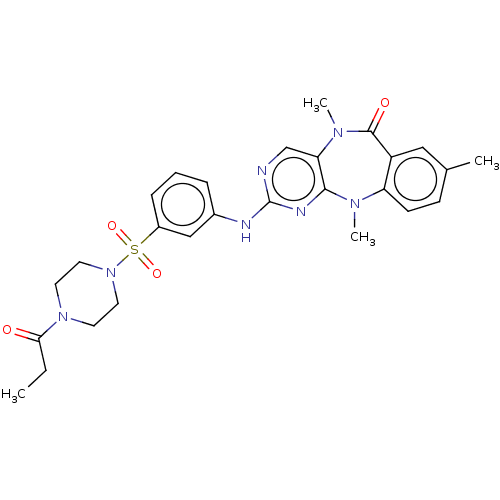
(CHEMBL3942643 | US11155556, No. 9)Show SMILES CCC(=O)N1CCN(CC1)S(=O)(=O)c1cccc(Nc2ncc3N(C)C(=O)c4cc(C)ccc4N(C)c3n2)c1 Show InChI InChI=1S/C27H31N7O4S/c1-5-24(35)33-11-13-34(14-12-33)39(37,38)20-8-6-7-19(16-20)29-27-28-17-23-25(30-27)31(3)22-10-9-18(2)15-21(22)26(36)32(23)4/h6-10,15-17H,5,11-14H2,1-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150175
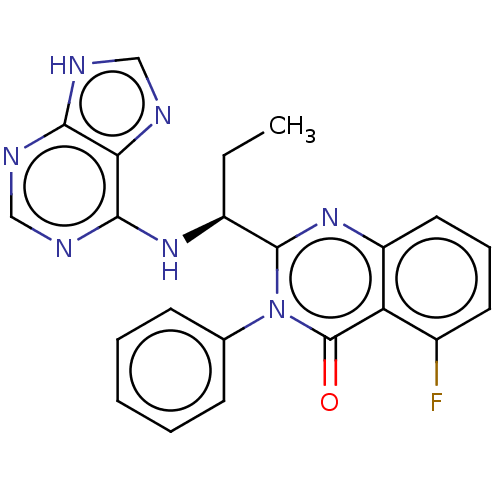
(US8980901, 107 | US9149477, Compound 107)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50193031
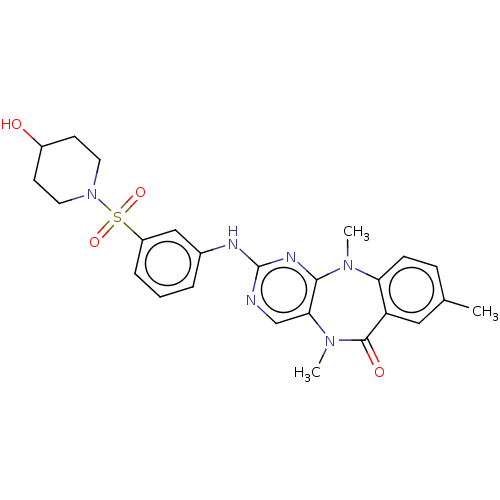
(CHEMBL3959351 | US11155556, No. 12)Show SMILES CN1c2ccc(C)cc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(=O)(=O)N3CCC(O)CC3)nc12 Show InChI InChI=1S/C25H28N6O4S/c1-16-7-8-21-20(13-16)24(33)30(3)22-15-26-25(28-23(22)29(21)2)27-17-5-4-6-19(14-17)36(34,35)31-11-9-18(32)10-12-31/h4-8,13-15,18,32H,9-12H2,1-3H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50193017
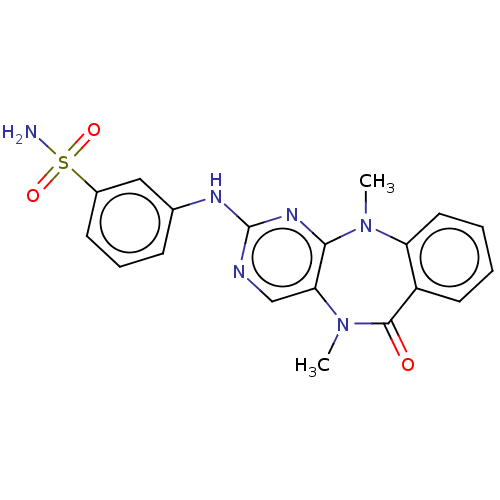
(CHEMBL3904942 | US11155556, No. 17)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C19H18N6O3S/c1-24-15-9-4-3-8-14(15)18(26)25(2)16-11-21-19(23-17(16)24)22-12-6-5-7-13(10-12)29(20,27)28/h3-11H,1-2H3,(H2,20,27,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora kinase A (E122 to K401 residues) expressed in mammalian expression system by Z'LYTE assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50193032

(CHEMBL3979343 | US11155556, No. 2)Show SMILES CN1c2ccc(C)cc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(=O)(=O)N3CCN(CC3)C(=O)C=C)nc12 Show InChI InChI=1S/C27H29N7O4S/c1-5-24(35)33-11-13-34(14-12-33)39(37,38)20-8-6-7-19(16-20)29-27-28-17-23-25(30-27)31(3)22-10-9-18(2)15-21(22)26(36)32(23)4/h5-10,15-17H,1,11-14H2,2-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50193013

(Duvelisib | INK-1147 | INK-1197 | IPI-145)Show SMILES C[C@H](Nc1ncnc2nc[nH]c12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H17ClN6O/c1-13(28-21-19-20(25-11-24-19)26-12-27-21)17-10-14-6-5-9-16(23)18(14)22(30)29(17)15-7-3-2-4-8-15/h2-13H,1H3,(H2,24,25,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50193017

(CHEMBL3904942 | US11155556, No. 17)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C19H18N6O3S/c1-24-15-9-4-3-8-14(15)18(26)25(2)16-11-21-19(23-17(16)24)22-12-6-5-7-13(10-12)29(20,27)28/h3-11H,1-2H3,(H2,20,27,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50193030

(CHEMBL3950648 | US11155556, No. 14)Show SMILES CN1c2ccc(C)cc2C(=O)N(C)c2cnc(Nc3ccc(cc3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C20H20N6O3S/c1-12-4-9-16-15(10-12)19(27)26(3)17-11-22-20(24-18(17)25(16)2)23-13-5-7-14(8-6-13)30(21,28)29/h4-11H,1-3H3,(H2,21,28,29)(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora kinase B (D25 to A303 residues) expressed in mammalian expression system by Z'LYTE assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50193017

(CHEMBL3904942 | US11155556, No. 17)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C19H18N6O3S/c1-24-15-9-4-3-8-14(15)18(26)25(2)16-11-21-19(23-17(16)24)22-12-6-5-7-13(10-12)29(20,27)28/h3-11H,1-2H3,(H2,20,27,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50193017

(CHEMBL3904942 | US11155556, No. 17)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C19H18N6O3S/c1-24-15-9-4-3-8-14(15)18(26)25(2)16-11-21-19(23-17(16)24)22-12-6-5-7-13(10-12)29(20,27)28/h3-11H,1-2H3,(H2,20,27,28)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora kinase B (D25 to A303 residues) expressed in mammalian expression system by Z'LYTE assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50337135
(4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C19H18N6O3S/c1-24-15-6-4-3-5-14(15)18(26)25(2)16-11-21-19(23-17(16)24)22-12-7-9-13(10-8-12)29(20,27)28/h3-11H,1-2H3,(H2,20,27,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora kinase A (E122 to K401 residues) expressed in mammalian expression system by Z'LYTE assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50193031

(CHEMBL3959351 | US11155556, No. 12)Show SMILES CN1c2ccc(C)cc2C(=O)N(C)c2cnc(Nc3cccc(c3)S(=O)(=O)N3CCC(O)CC3)nc12 Show InChI InChI=1S/C25H28N6O4S/c1-16-7-8-21-20(13-16)24(33)30(3)22-15-26-25(28-23(22)29(21)2)27-17-5-4-6-19(14-17)36(34,35)31-11-9-18(32)10-12-31/h4-8,13-15,18,32H,9-12H2,1-3H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50193014

(CHEMBL3942643 | US11155556, No. 9)Show SMILES CCC(=O)N1CCN(CC1)S(=O)(=O)c1cccc(Nc2ncc3N(C)C(=O)c4cc(C)ccc4N(C)c3n2)c1 Show InChI InChI=1S/C27H31N7O4S/c1-5-24(35)33-11-13-34(14-12-33)39(37,38)20-8-6-7-19(16-20)29-27-28-17-23-25(30-27)31(3)22-10-9-18(2)15-21(22)26(36)32(23)4/h6-10,15-17H,5,11-14H2,1-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50337135

(4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C19H18N6O3S/c1-24-15-6-4-3-5-14(15)18(26)25(2)16-11-21-19(23-17(16)24)22-12-7-9-13(10-8-12)29(20,27)28/h3-11H,1-2H3,(H2,20,27,28)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora kinase B (D25 to A303 residues) expressed in mammalian expression system by Z'LYTE assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50337135

(4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)S(N)(=O)=O)nc12 Show InChI InChI=1S/C19H18N6O3S/c1-24-15-6-4-3-5-14(15)18(26)25(2)16-11-21-19(23-17(16)24)22-12-7-9-13(10-8-12)29(20,27)28/h3-11H,1-2H3,(H2,20,27,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay |
ACS Med Chem Lett 7: 908-912 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00209
BindingDB Entry DOI: 10.7270/Q27P91B0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data