 Found 632 hits with Last Name = 'zheng' and Initial = 'p'
Found 632 hits with Last Name = 'zheng' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50110577
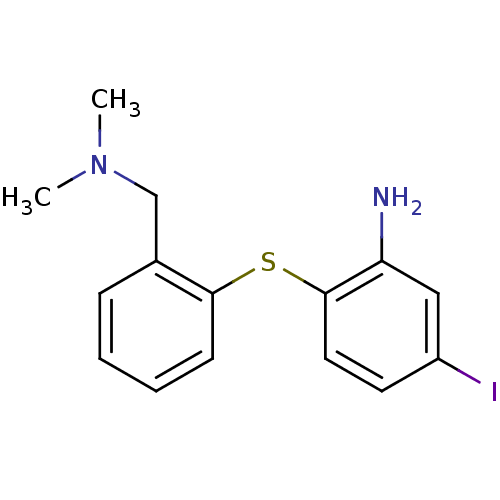
(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IPT uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50110577

(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity to SERT (unknown origin) |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50425189
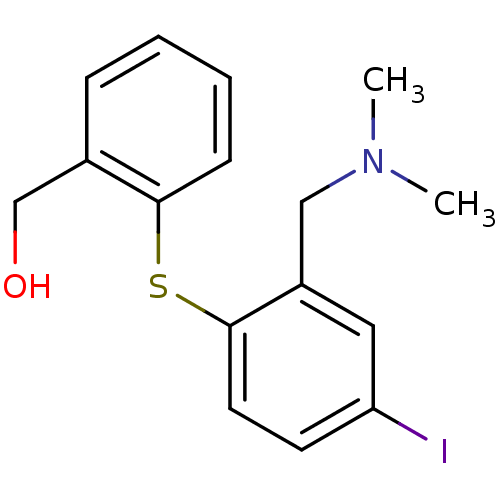
(CHEMBL2314217)Show InChI InChI=1S/C16H18INOS/c1-18(2)10-13-9-14(17)7-8-16(13)20-15-6-4-3-5-12(15)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IDAM uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50425187
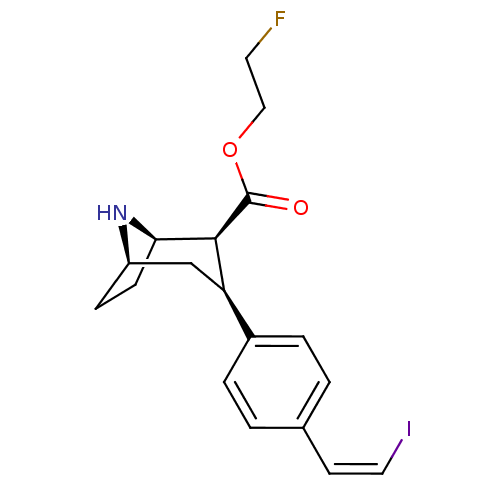
(CHEMBL2314215)Show SMILES FCCOC(=O)[C@@H]1[C@H]2CC[C@H](C[C@@H]1c1ccc(\C=C/I)cc1)N2 |r,TLB:13:12:22:8.9,THB:4:6:22:8.9| Show InChI InChI=1S/C18H21FINO2/c19-8-10-23-18(22)17-15(11-14-5-6-16(17)21-14)13-3-1-12(2-4-13)7-9-20/h1-4,7,9,14-17,21H,5-6,8,10-11H2/b9-7-/t14-,15-,16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity to SERT (unknown origin) |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50073436
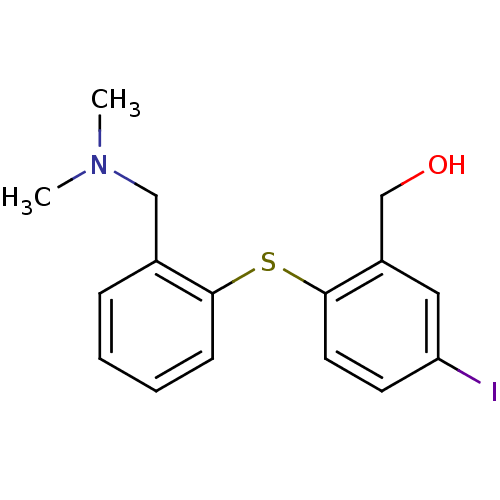
((2-(2-((dimethylamino)methyl)phenylthio)-5-iodophe...)Show InChI InChI=1S/C16H18INOS/c1-18(2)10-12-5-3-4-6-15(12)20-16-8-7-14(17)9-13(16)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity to SERT (unknown origin) |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50073436

((2-(2-((dimethylamino)methyl)phenylthio)-5-iodophe...)Show InChI InChI=1S/C16H18INOS/c1-18(2)10-12-5-3-4-6-15(12)20-16-8-7-14(17)9-13(16)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IPT uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50301022
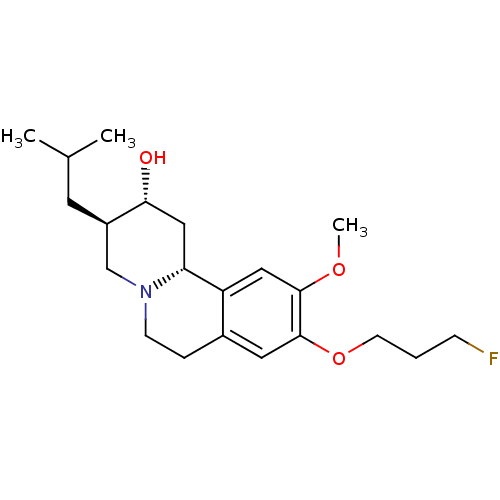
((+)-9-fluoropropyl-dihydrotetrabenazine | CHEMBL57...)Show SMILES COc1cc2[C@H]3C[C@@H](O)[C@H](CC(C)C)CN3CCc2cc1OCCCF |r| Show InChI InChI=1S/C21H32FNO3/c1-14(2)9-16-13-23-7-5-15-10-21(26-8-4-6-22)20(25-3)11-17(15)18(23)12-19(16)24/h10-11,14,16,18-19,24H,4-9,12-13H2,1-3H3/t16-,18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodovinyl-TBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM25870
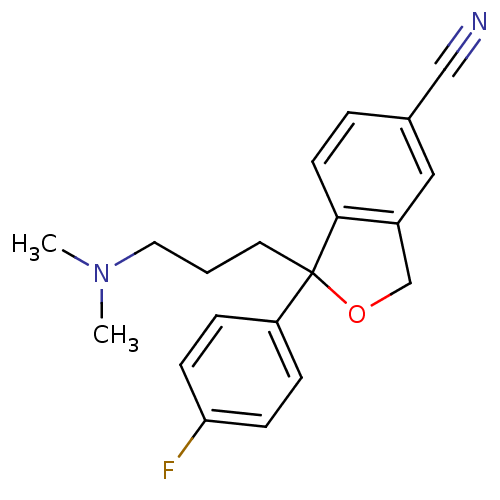
(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IDAM uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50425190
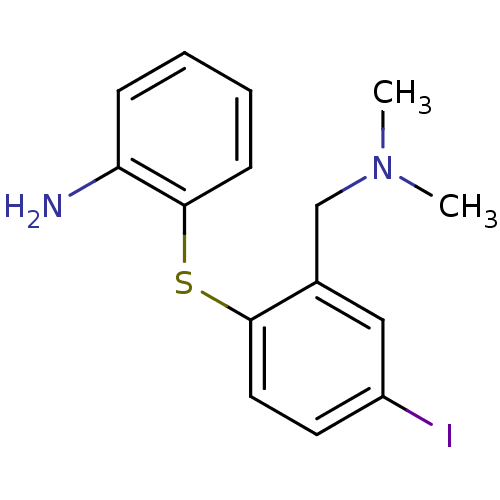
(CHEMBL2314216)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-9-12(16)7-8-14(11)19-15-6-4-3-5-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IDAM uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50301021
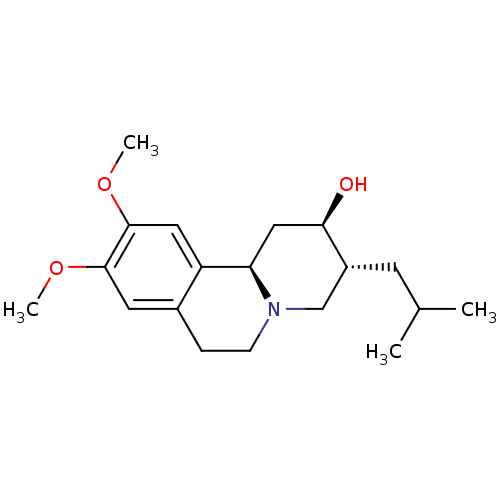
((+)-dihydrotetrabenzaine | CHEMBL576222 | US110532...)Show SMILES COc1cc2CCN3C[C@@H](CC(C)C)[C@H](O)C[C@@H]3c2cc1OC |r| Show InChI InChI=1S/C19H29NO3/c1-12(2)7-14-11-20-6-5-13-8-18(22-3)19(23-4)9-15(13)16(20)10-17(14)21/h8-9,12,14,16-17,21H,5-7,10-11H2,1-4H3/t14-,16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodovinyl-TBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50344439
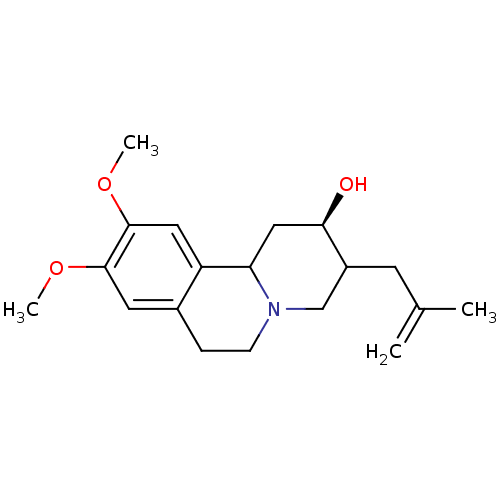
(CHEMBL1780005 | rac-(2R)-9,10-dimethoxy-3-(2-methy...)Show SMILES COc1cc2CCN3CC(CC(C)=C)[C@H](O)CC3c2cc1OC |r| Show InChI InChI=1S/C19H27NO3/c1-12(2)7-14-11-20-6-5-13-8-18(22-3)19(23-4)9-15(13)16(20)10-17(14)21/h8-9,14,16-17,21H,1,5-7,10-11H2,2-4H3/t14?,16?,17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodovinyl-TBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50344443
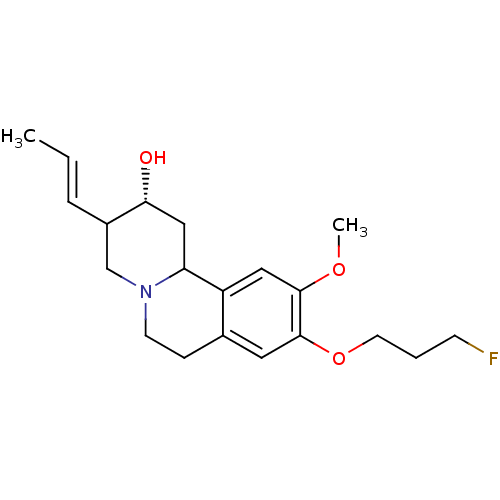
(CHEMBL1780009 | rac-(2R)-9-(3-fluoropropoxy)-10-me...)Show SMILES COc1cc2C3C[C@@H](O)C(CN3CCc2cc1OCCCF)\C=C\C |r| Show InChI InChI=1S/C20H28FNO3/c1-3-5-15-13-22-8-6-14-10-20(25-9-4-7-21)19(24-2)11-16(14)17(22)12-18(15)23/h3,5,10-11,15,17-18,23H,4,6-9,12-13H2,1-2H3/b5-3+/t15?,17?,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [18F](+)-FP-DTBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50344440
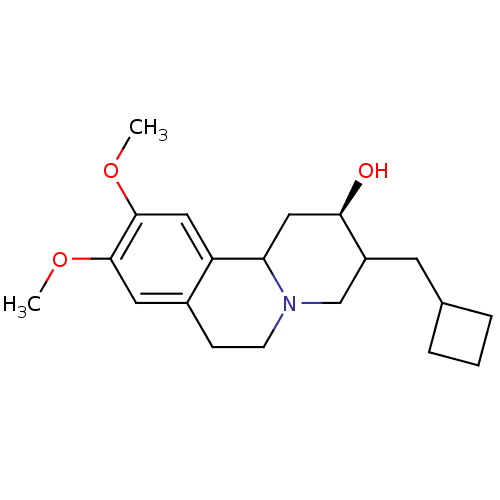
(CHEMBL1780006 | rac-(2R)-3-(cyclobutylmethyl)-9,10...)Show SMILES COc1cc2CCN3CC(CC4CCC4)[C@H](O)CC3c2cc1OC |r| Show InChI InChI=1S/C20H29NO3/c1-23-19-9-14-6-7-21-12-15(8-13-4-3-5-13)18(22)11-17(21)16(14)10-20(19)24-2/h9-10,13,15,17-18,22H,3-8,11-12H2,1-2H3/t15?,17?,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodovinyl-TBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50344442

(CHEMBL1780008 | rac-(2R)-3-(cyclohexylmethyl)-9,10...)Show SMILES COc1cc2CCN3CC(CC4CCCCC4)[C@H](O)CC3c2cc1OC |r| Show InChI InChI=1S/C22H33NO3/c1-25-21-11-16-8-9-23-14-17(10-15-6-4-3-5-7-15)20(24)13-19(23)18(16)12-22(21)26-2/h11-12,15,17,19-20,24H,3-10,13-14H2,1-2H3/t17?,19?,20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodovinyl-TBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50344441
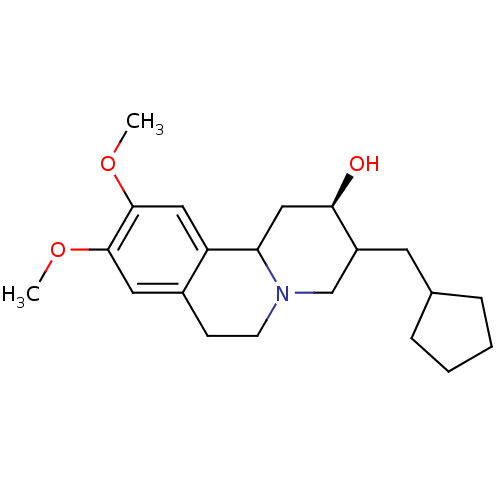
(CHEMBL1780007 | rac-(2R)-3-(cyclopentylmethyl)-9,1...)Show SMILES COc1cc2CCN3CC(CC4CCCC4)[C@H](O)CC3c2cc1OC |r| Show InChI InChI=1S/C21H31NO3/c1-24-20-10-15-7-8-22-13-16(9-14-5-3-4-6-14)19(23)12-18(22)17(15)11-21(20)25-2/h10-11,14,16,18-19,23H,3-9,12-13H2,1-2H3/t16?,18?,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodovinyl-TBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Synaptic vesicular amine transporter
(Rattus norvegicus (Rat)) | BDBM50344438

(CHEMBL1780004 | rac-(2R)-3-allyl-9,10-dimethoxy-2,...)Show InChI InChI=1S/C18H25NO3/c1-4-5-13-11-19-7-6-12-8-17(21-2)18(22-3)9-14(12)15(19)10-16(13)20/h4,8-9,13,15-16,20H,1,5-7,10-11H2,2-3H3/t13?,15?,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Displacement of [18F](+)-FP-DTBZ from VMAT2 in rat striatal homogenate after 60 mins by gamma counting |
Bioorg Med Chem Lett 21: 3435-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.113
BindingDB Entry DOI: 10.7270/Q289166R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50260360
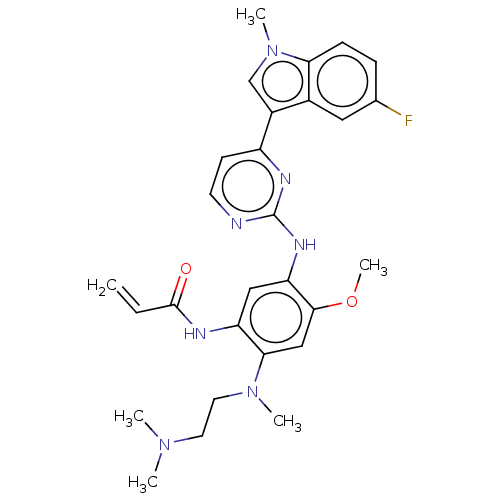
(CHEMBL4103912)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccc(F)cc12 Show InChI InChI=1S/C28H32FN7O2/c1-7-27(37)31-22-15-23(26(38-6)16-25(22)35(4)13-12-34(2)3)33-28-30-11-10-21(32-28)20-17-36(5)24-9-8-18(29)14-19(20)24/h7-11,14-17H,1,12-13H2,2-6H3,(H,31,37)(H,30,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) using substrate-biotin by ELISA-based mobility shift assay |
Eur J Med Chem 163: 367-380 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.069
BindingDB Entry DOI: 10.7270/Q2BP0677 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50260360

(CHEMBL4103912)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccc(F)cc12 Show InChI InChI=1S/C28H32FN7O2/c1-7-27(37)31-22-15-23(26(38-6)16-25(22)35(4)13-12-34(2)3)33-28-30-11-10-21(32-28)20-17-36(5)24-9-8-18(29)14-19(20)24/h7-11,14-17H,1,12-13H2,2-6H3,(H,31,37)(H,30,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) using substrate-biotin by ELISA-based mobility shift assay |
Eur J Med Chem 163: 367-380 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.069
BindingDB Entry DOI: 10.7270/Q2BP0677 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606494

(CHEMBL5220645)Show SMILES CCC(=O)Nc1cccc(c1)-c1c([nH]c2ncnc(OC(C)C)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606493

(CHEMBL5218754)Show SMILES CCCCOc1ncnc2[nH]c(c(-c3cccc(NC(=O)CC)c3)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606495

(CHEMBL5220529)Show SMILES CCC(=O)Nc1cccc(c1)-c1c([nH]c2ncnc(OCC(C)C)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606494

(CHEMBL5220645)Show SMILES CCC(=O)Nc1cccc(c1)-c1c([nH]c2ncnc(OC(C)C)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606493

(CHEMBL5218754)Show SMILES CCCCOc1ncnc2[nH]c(c(-c3cccc(NC(=O)CC)c3)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM538108
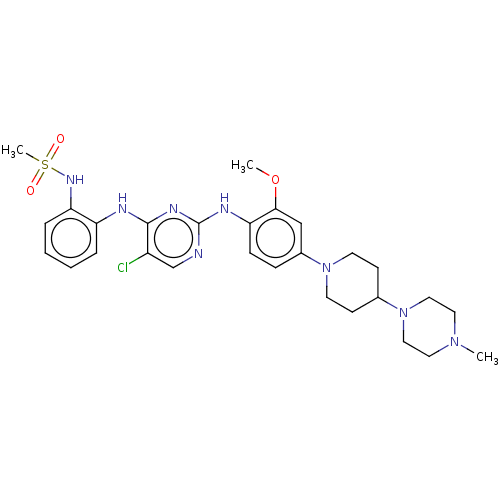
(US11253516, Example 10)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606495

(CHEMBL5220529)Show SMILES CCC(=O)Nc1cccc(c1)-c1c([nH]c2ncnc(OCC(C)C)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM538135
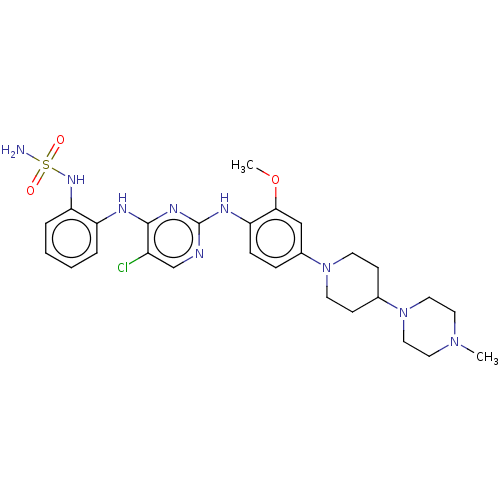
(US11253516, Example 37)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(N)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606497

(CHEMBL5218879)Show SMILES OCCCCc1ccc(cc1)-c1nc(c([nH]1)-c1ccnc2NC(Cc12)c1ccccc1)-c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606496

(CHEMBL5220061)Show SMILES OCCc1ccc(cc1)-c1nc(c([nH]1)-c1ccnc2NC(Cc12)c1ccccc1)-c1ccc(F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606492

(CHEMBL5218797)Show SMILES CCC(=O)Nc1cccc(c1)-c1c([nH]c2ncn(C3CCCCC3)c(=O)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50606492

(CHEMBL5218797)Show SMILES CCC(=O)Nc1cccc(c1)-c1c([nH]c2ncn(C3CCCCC3)c(=O)c12)-c1ccc(cc1)N1CCN(C)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50406868

(BIBW2992 | CHEMBL2347958)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 154: 29-43 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.006
BindingDB Entry DOI: 10.7270/Q2S46VJX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM538120
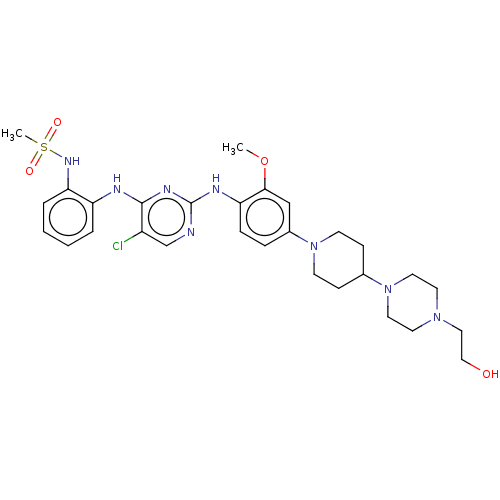
(US11253516, Example 22)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCC(CC1)N1CCN(CCO)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112995
BindingDB Entry DOI: 10.7270/Q2DN494X |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50251300

(CHEMBL4066684)Show SMILES Cc1cc(=O)n(nc1C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)c(F)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H17F2N5O3/c1-14-12-22(33)32(17-5-2-15(26)3-6-17)31-23(14)25(34)30-16-4-7-21(19(27)13-16)35-20-9-11-29-24-18(20)8-10-28-24/h2-13H,1H3,(H,28,29)(H,30,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy, Jiangxi Science and Technology Normal University, Nanchang 330013, PR China. Electronic address: tangqidongcn@126.com.
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Eur J Med Chem 133: 97-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.045
BindingDB Entry DOI: 10.7270/Q2K35X3B |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50152685
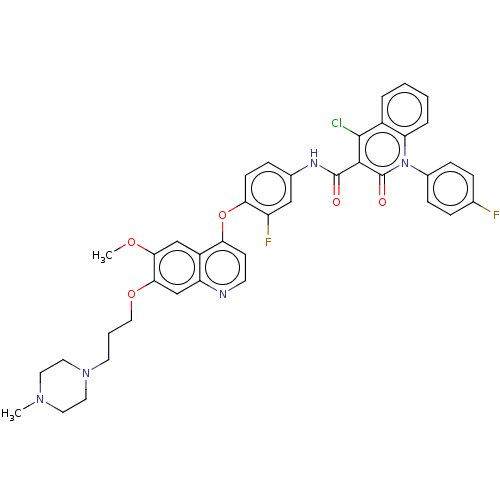
(CHEMBL3780676)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4c(Cl)c5ccccc5n(-c5ccc(F)cc5)c4=O)cc3F)ccnc2cc1OCCCN1CCN(C)CC1 Show InChI InChI=1S/C40H36ClF2N5O5/c1-46-17-19-47(20-18-46)16-5-21-52-36-24-31-29(23-35(36)51-2)33(14-15-44-31)53-34-13-10-26(22-30(34)43)45-39(49)37-38(41)28-6-3-4-7-32(28)48(40(37)50)27-11-8-25(42)9-12-27/h3-4,6-15,22-24H,5,16-21H2,1-2H3,(H,45,49) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of cMet (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1794-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.037
BindingDB Entry DOI: 10.7270/Q2TT4ST4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50464538
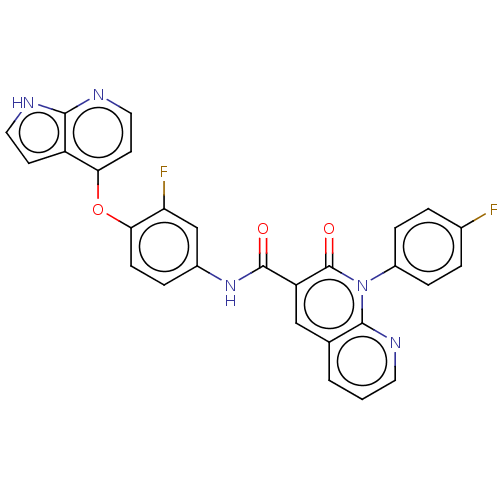
(CHEMBL4289577)Show SMILES Fc1ccc(cc1)-n1c2ncccc2cc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1=O Show InChI InChI=1S/C28H17F2N5O3/c29-17-3-6-19(7-4-17)35-26-16(2-1-11-33-26)14-21(28(35)37)27(36)34-18-5-8-24(22(30)15-18)38-23-10-13-32-25-20(23)9-12-31-25/h1-15H,(H,31,32)(H,34,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Flt-3 (unknown origin) using poly(Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Eur J Med Chem 143: 266-275 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.034
BindingDB Entry DOI: 10.7270/Q2JW8HJZ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50251300

(CHEMBL4066684)Show SMILES Cc1cc(=O)n(nc1C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)c(F)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H17F2N5O3/c1-14-12-22(33)32(17-5-2-15(26)3-6-17)31-23(14)25(34)30-16-4-7-21(19(27)13-16)35-20-9-11-29-24-18(20)8-10-28-24/h2-13H,1H3,(H,28,29)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy, Jiangxi Science and Technology Normal University, Nanchang 330013, PR China. Electronic address: tangqidongcn@126.com.
Curated by ChEMBL
| Assay Description
Inhibition of Flt-3 (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Eur J Med Chem 133: 97-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.045
BindingDB Entry DOI: 10.7270/Q2K35X3B |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50021574
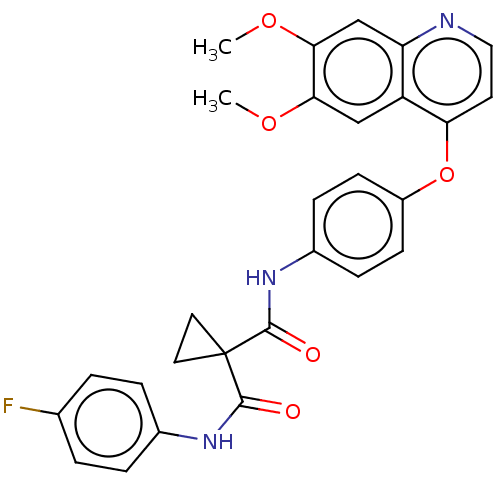
(BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3)c2cc1OC Show InChI InChI=1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by caliper mobility shift assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126848
BindingDB Entry DOI: 10.7270/Q2CV4N8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by addition of substrate by mobility shi... |
Bioorg Med Chem 24: 1495-503 (2016)
Article DOI: 10.1016/j.bmc.2016.02.017
BindingDB Entry DOI: 10.7270/Q2KS6TCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis |
Eur J Med Chem 143: 266-275 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.034
BindingDB Entry DOI: 10.7270/Q2JW8HJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate measured after 10 mins by mobility shift assay |
Bioorg Med Chem 25: 3148-3157 (2017)
Article DOI: 10.1016/j.bmc.2017.04.001
BindingDB Entry DOI: 10.7270/Q24X5B6W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50154813
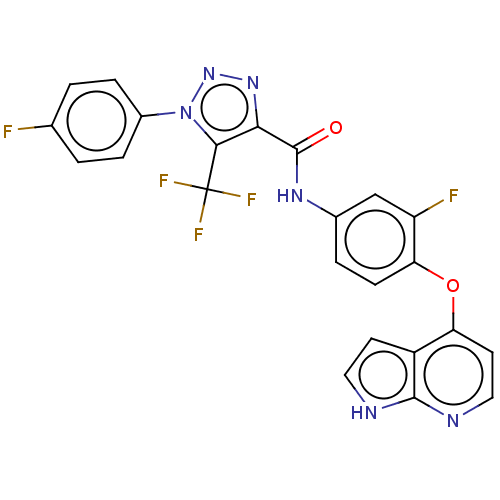
(CHEMBL3774451)Show SMILES Fc1ccc(cc1)-n1nnc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1C(F)(F)F Show InChI InChI=1S/C23H13F5N6O2/c24-12-1-4-14(5-2-12)34-20(23(26,27)28)19(32-33-34)22(35)31-13-3-6-18(16(25)11-13)36-17-8-10-30-21-15(17)7-9-29-21/h1-11H,(H,29,30)(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of cMet (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1680-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.059
BindingDB Entry DOI: 10.7270/Q2FF3V7R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-MET (unknown origin) using FAM-labeled peptide as substrate incubated for 10 mins followed by substrate addition by HTRF assay |
Eur J Med Chem 158: 201-213 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.066
BindingDB Entry DOI: 10.7270/Q2H134QT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of cMet (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1794-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.037
BindingDB Entry DOI: 10.7270/Q2TT4ST4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50152684

(CHEMBL3781716)Show SMILES COc1cc2c(Oc3ccc(NC(=O)c4c(Cl)c5ccccc5n(-c5ccccc5)c4=O)cc3F)ccnc2cc1OCCCN1CCN(C)CC1 Show InChI InChI=1S/C40H37ClFN5O5/c1-45-18-20-46(21-19-45)17-8-22-51-36-25-31-29(24-35(36)50-2)33(15-16-43-31)52-34-14-13-26(23-30(34)42)44-39(48)37-38(41)28-11-6-7-12-32(28)47(40(37)49)27-9-4-3-5-10-27/h3-7,9-16,23-25H,8,17-22H2,1-2H3,(H,44,48) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of cMet (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1794-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.037
BindingDB Entry DOI: 10.7270/Q2TT4ST4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50464538

(CHEMBL4289577)Show SMILES Fc1ccc(cc1)-n1c2ncccc2cc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1=O Show InChI InChI=1S/C28H17F2N5O3/c29-17-3-6-19(7-4-17)35-26-16(2-1-11-33-26)14-21(28(35)37)27(36)34-18-5-8-24(22(30)15-18)38-23-10-13-32-25-20(23)9-12-31-25/h1-15H,(H,31,32)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta (unknown origin) using poly(Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Eur J Med Chem 143: 266-275 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.034
BindingDB Entry DOI: 10.7270/Q2JW8HJZ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50464538

(CHEMBL4289577)Show SMILES Fc1ccc(cc1)-n1c2ncccc2cc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1=O Show InChI InChI=1S/C28H17F2N5O3/c29-17-3-6-19(7-4-17)35-26-16(2-1-11-33-26)14-21(28(35)37)27(36)34-18-5-8-24(22(30)15-18)38-23-10-13-32-25-20(23)9-12-31-25/h1-15H,(H,31,32)(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis |
Eur J Med Chem 143: 266-275 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.034
BindingDB Entry DOI: 10.7270/Q2JW8HJZ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of cMet (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1680-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.059
BindingDB Entry DOI: 10.7270/Q2FF3V7R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50154813

(CHEMBL3774451)Show SMILES Fc1ccc(cc1)-n1nnc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1C(F)(F)F Show InChI InChI=1S/C23H13F5N6O2/c24-12-1-4-14(5-2-12)34-20(23(26,27)28)19(32-33-34)22(35)31-13-3-6-18(16(25)11-13)36-17-8-10-30-21-15(17)7-9-29-21/h1-11H,(H,29,30)(H,31,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science and Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1680-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.059
BindingDB Entry DOI: 10.7270/Q2FF3V7R |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50263263

(CHEMBL4073443)Show SMILES Cc1cc(Nc2cc(N)ncn2)c(=O)n2c1C(=O)NC21CCCCC1 Show InChI InChI=1S/C17H20N6O2/c1-10-7-11(21-13-8-12(18)19-9-20-13)16(25)23-14(10)15(24)22-17(23)5-3-2-4-6-17/h7-9H,2-6H2,1H3,(H,22,24)(H3,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged MNK2 (1 to 465 residues) expressed in baculovirus expression system using TATKSGSTTKNR as subst... |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.02.007
BindingDB Entry DOI: 10.7270/Q2V128D5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmacy, Jiangxi Science and Technology Normal University, Nanchang 330013, PR China. Electronic address: tangqidongcn@126.com.
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay |
Eur J Med Chem 133: 97-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.045
BindingDB Entry DOI: 10.7270/Q2K35X3B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data