

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase-1 (Homo sapiens (Human)) | BDBM50465984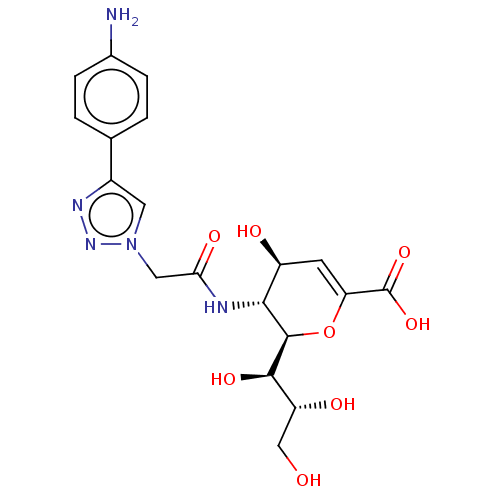 (CHEMBL4278858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Competitive inhibition of human His6-tagged NEU1 expressed in HEK293 cells using 4MU-NANA as substrate preincubated with substrate for 15 mins and me... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-1 (Homo sapiens (Human)) | BDBM50465968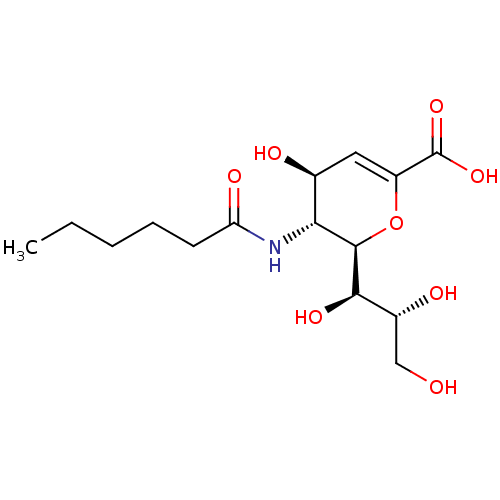 (CHEMBL4291908) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Competitive inhibition of human His6-tagged NEU1 expressed in HEK293 cells using 4MU-NANA as substrate preincubated with substrate for 15 mins and me... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-1 (Homo sapiens (Human)) | BDBM50465979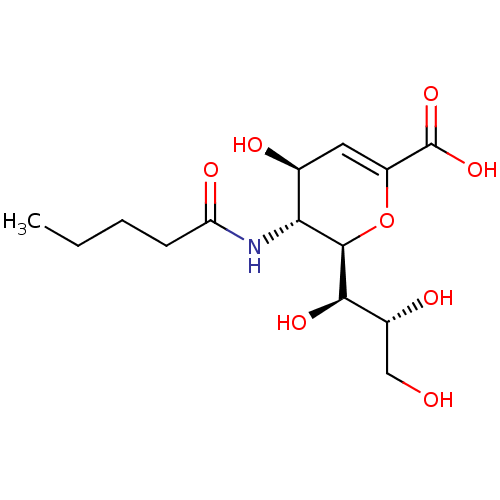 (CHEMBL4281290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Competitive inhibition of human His6-tagged NEU1 expressed in HEK293 cells using 4MU-NANA as substrate preincubated with substrate for 15 mins and me... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-4 (Homo sapiens (Human)) | BDBM50270464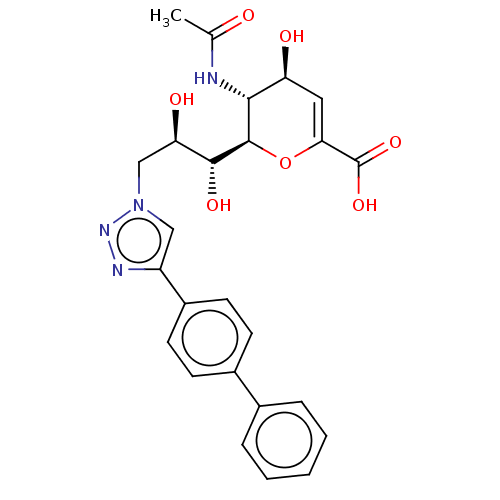 (CHEMBL4076203) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU4 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-3 (Homo sapiens (Human)) | BDBM50270464 (CHEMBL4076203) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU3 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-3 (Homo sapiens (Human)) | BDBM50270441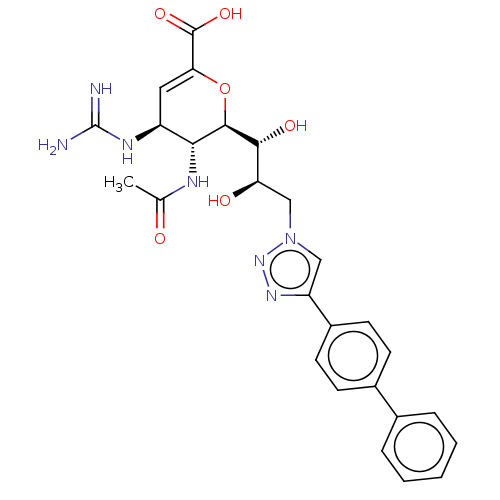 (CHEMBL4099818) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU3 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-3 (Homo sapiens (Human)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU3 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-1 (Homo sapiens (Human)) | BDBM50270454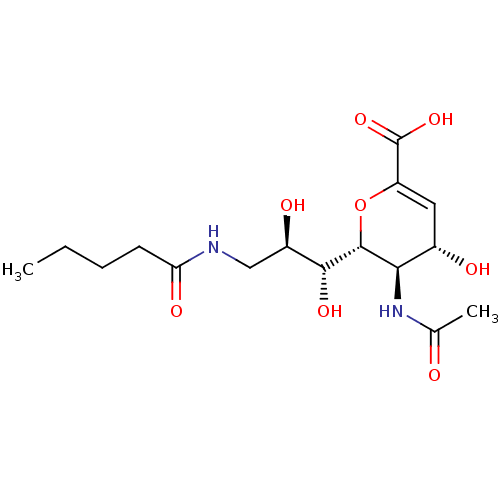 (CHEMBL4076483) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Non-competitive inhibition of human His6-tagged NEU1 expressed in HEK293 cells using 4MU-NANA as substrate preincubated with substrate for 15 mins an... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50465983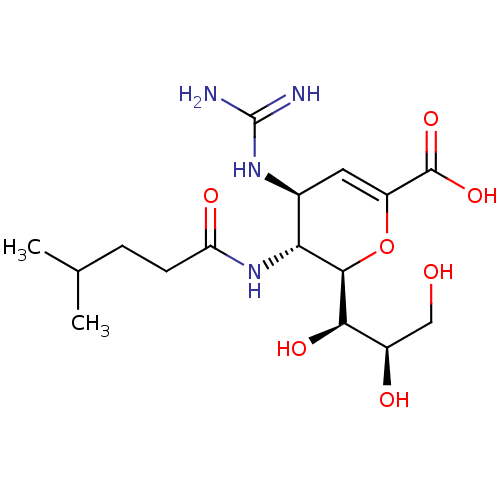 (CHEMBL4277689) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal MBP-fused human NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated with substrate for 1... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-3 (Homo sapiens (Human)) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU3 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50465984 (CHEMBL4278858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Non-competitive inhibition of N-terminal MBP-fused human NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated with substrate f... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50465985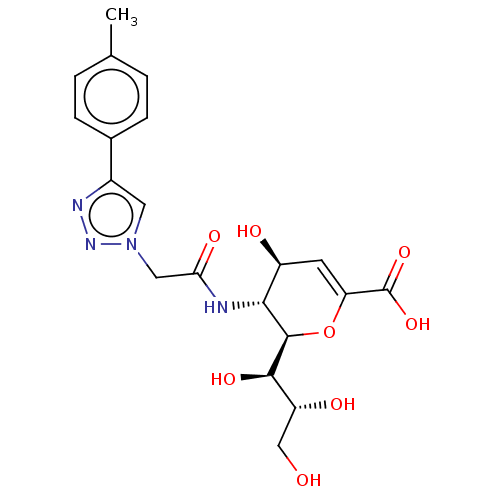 (CHEMBL4280792) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Non-competitive inhibition of N-terminal MBP-fused human NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated with substrate f... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-4 (Homo sapiens (Human)) | BDBM50270441 (CHEMBL4099818) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU4 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-4 (Homo sapiens (Human)) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU4 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-1 (Homo sapiens (Human)) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Competitive inhibition of human His6-tagged NEU1 expressed in HEK293 cells using 4MU-NANA as substrate preincubated with substrate for 15 mins and me... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50270441 (CHEMBL4099818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Non-competitive inhibition of N-terminal MBP-fused human NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated with substrate f... | J Med Chem 61: 11261-11279 (2018) Article DOI: 10.1021/acs.jmedchem.8b01411 BindingDB Entry DOI: 10.7270/Q2GF0X5V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-4 (Homo sapiens (Human)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU4 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50270464 (CHEMBL4076203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human N-terminal MBP-fused NEU2 expressed in Escherichia coli using 4MU-NANA as substrate preincubated for 15 mins followed by substrat... | J Med Chem 61: 1990-2008 (2018) Article DOI: 10.1021/acs.jmedchem.7b01574 BindingDB Entry DOI: 10.7270/Q2XS5XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50399676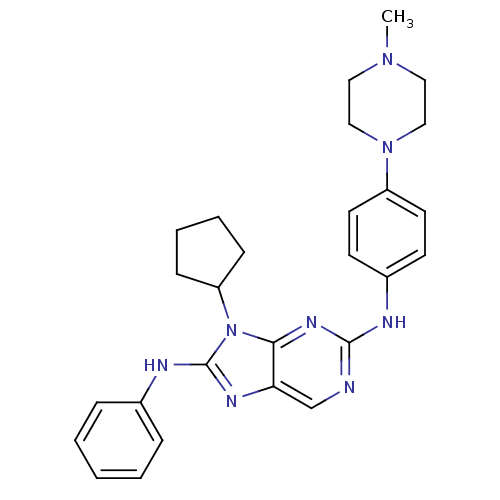 (CHEMBL2178352 | US9096601, 8-26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by radiometric kinase assay | J Med Chem 55: 10685-99 (2012) Article DOI: 10.1021/jm301365e BindingDB Entry DOI: 10.7270/Q2251KBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50399676 (CHEMBL2178352 | US9096601, 8-26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR by radiometric kinase assay | J Med Chem 55: 10685-99 (2012) Article DOI: 10.1021/jm301365e BindingDB Entry DOI: 10.7270/Q2251KBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50386751 (CHEMBL2046884) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human FLT3 | J Med Chem 55: 3852-66 (2012) Article DOI: 10.1021/jm300042x BindingDB Entry DOI: 10.7270/Q2M61M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50386750 (CHEMBL2046699) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human FLT3 | J Med Chem 55: 3852-66 (2012) Article DOI: 10.1021/jm300042x BindingDB Entry DOI: 10.7270/Q2M61M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50502595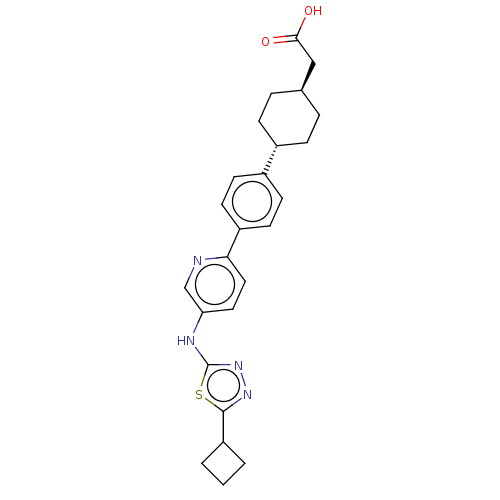 (CHEMBL4554065) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human his-tagged DGAT1 expressed in Sf9 insect cells using oleoyl-CoA and diolein as substrates incubated for 30 mins by LC... | ACS Med Chem Lett 10: 1128-1133 (2019) Article DOI: 10.1021/acsmedchemlett.9b00117 BindingDB Entry DOI: 10.7270/Q28W3HJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50386748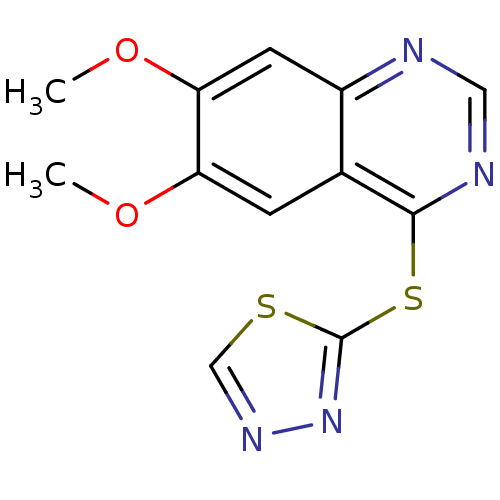 (CHEMBL2046726) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human FLT3 | J Med Chem 55: 3852-66 (2012) Article DOI: 10.1021/jm300042x BindingDB Entry DOI: 10.7270/Q2M61M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50386749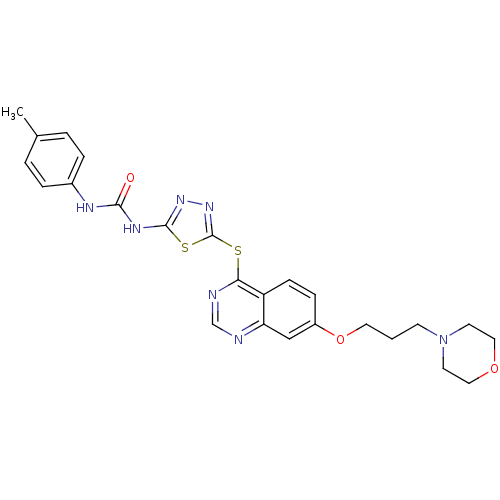 (CHEMBL2046883) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human FLT3 | J Med Chem 55: 3852-66 (2012) Article DOI: 10.1021/jm300042x BindingDB Entry DOI: 10.7270/Q2M61M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399676 (CHEMBL2178352 | US9096601, 8-26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR2 by radiometric kinase assay | J Med Chem 55: 10685-99 (2012) Article DOI: 10.1021/jm301365e BindingDB Entry DOI: 10.7270/Q2251KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50399676 (CHEMBL2178352 | US9096601, 8-26) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human recombinant ERBB4 by radiometric kinase assay | J Med Chem 55: 10685-99 (2012) Article DOI: 10.1021/jm301365e BindingDB Entry DOI: 10.7270/Q2251KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50502590 (CHEMBL4434994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human his-tagged DGAT1 expressed in Sf9 insect cells using oleoyl-CoA and diolein as substrates incubated for 30 mins by LC... | ACS Med Chem Lett 10: 1128-1133 (2019) Article DOI: 10.1021/acsmedchemlett.9b00117 BindingDB Entry DOI: 10.7270/Q28W3HJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50502586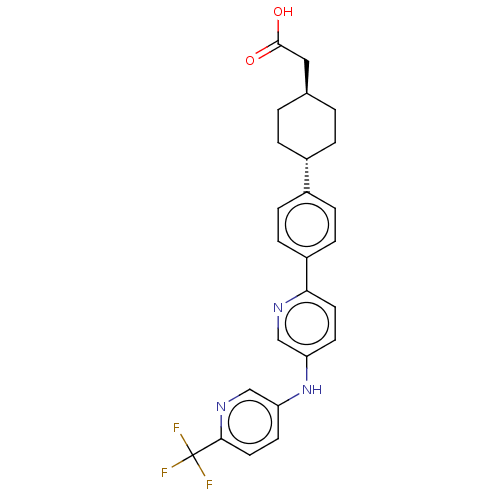 (LCQ-908-NXA | LCQ-908NXA | LCQ908-NXA | Pradigasta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human his-tagged DGAT1 expressed in Sf9 insect cells using oleoyl-CoA and diolein as substrates incubated for 30 mins by LC... | ACS Med Chem Lett 10: 1128-1133 (2019) Article DOI: 10.1021/acsmedchemlett.9b00117 BindingDB Entry DOI: 10.7270/Q28W3HJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50399676 (CHEMBL2178352 | US9096601, 8-26) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human recombinant ERBB2 by radiometric kinase assay | J Med Chem 55: 10685-99 (2012) Article DOI: 10.1021/jm301365e BindingDB Entry DOI: 10.7270/Q2251KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50502585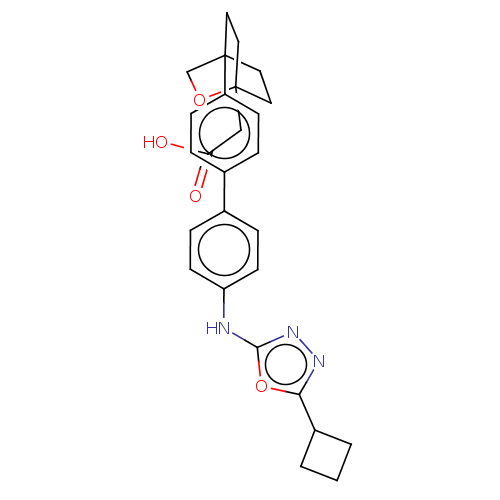 (CHEMBL4446248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human his-tagged DGAT1 expressed in Sf9 insect cells using oleoyl-CoA and diolein as substrates incubated for 30 mins by LC... | ACS Med Chem Lett 10: 1128-1133 (2019) Article DOI: 10.1021/acsmedchemlett.9b00117 BindingDB Entry DOI: 10.7270/Q28W3HJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494592 (US10988482, Compound 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494590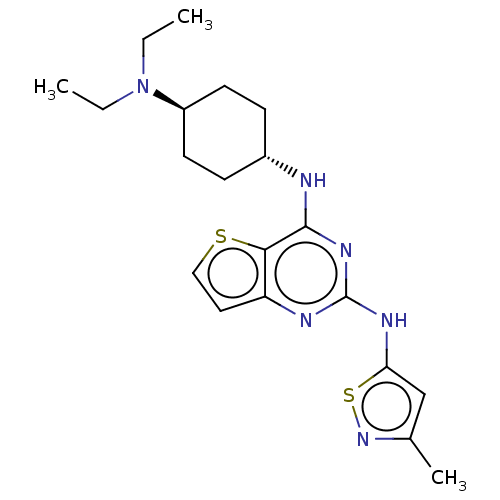 (US10988482, Compound 94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494591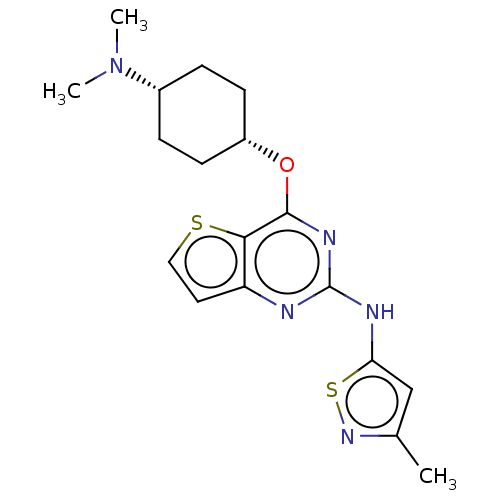 (US10988482, Compound 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494587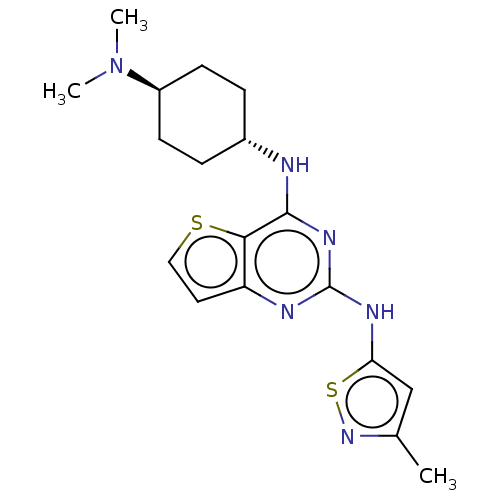 (US10988482, Compound 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494579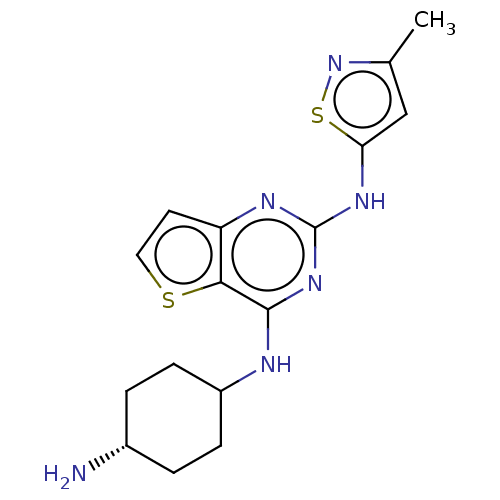 (US10988482, Compound 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494575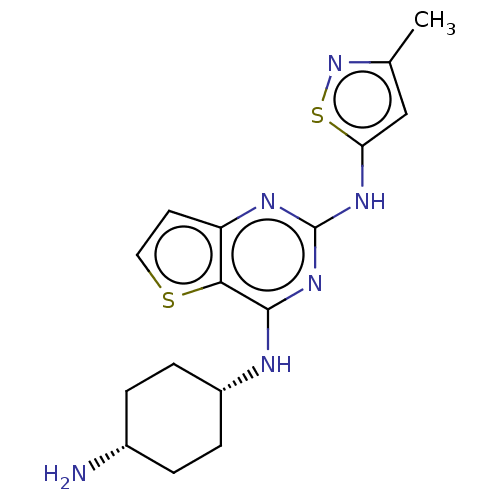 (US10988482, Compound 79 | US10988482, Compound 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494574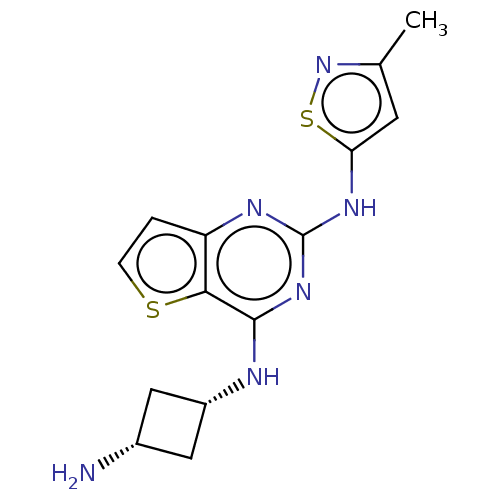 (US10988482, Compound 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494573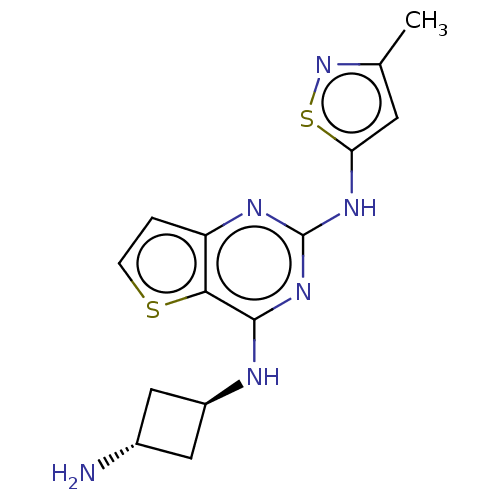 (US10988482, Compound 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494555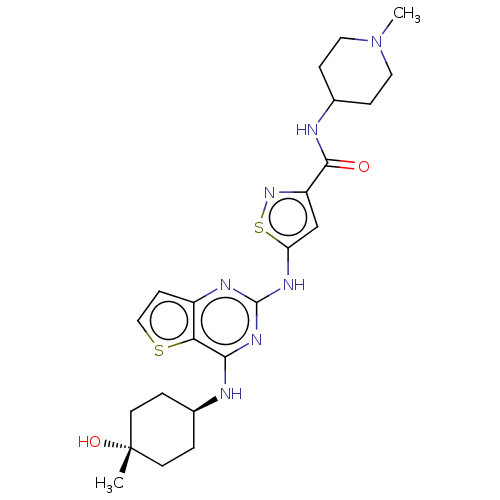 (US10988482, Compound 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494596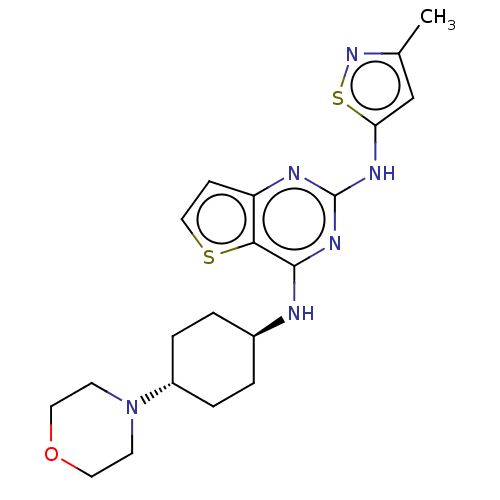 (US10988482, Compound 100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494595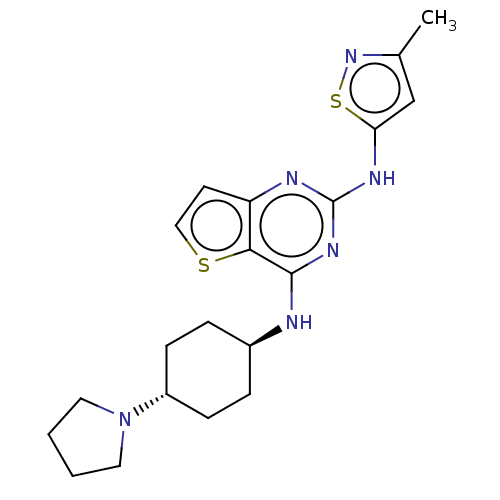 (US10988482, Compound 99) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM493932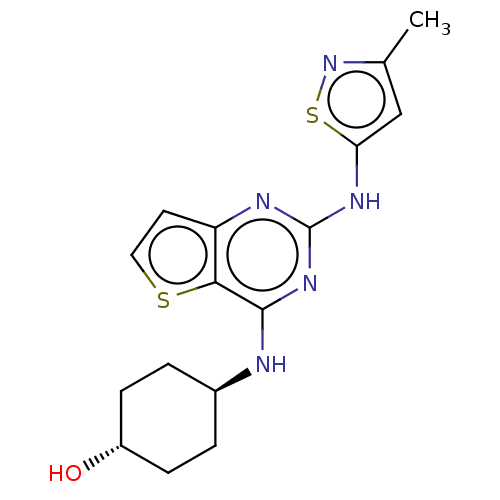 (US10988482, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494210 (US10988482, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494240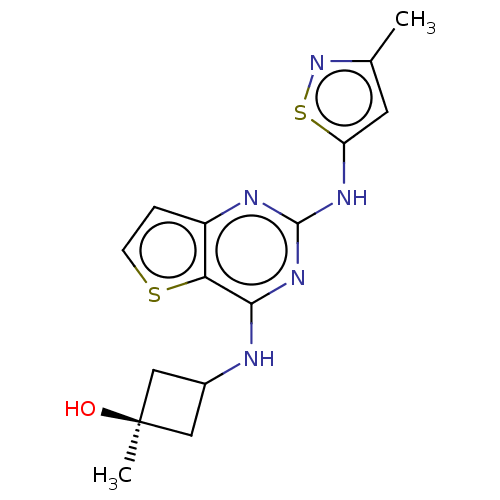 (US10988482, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494334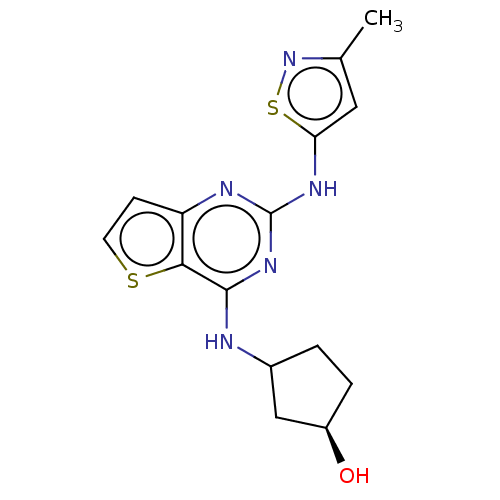 (US10988482, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM494435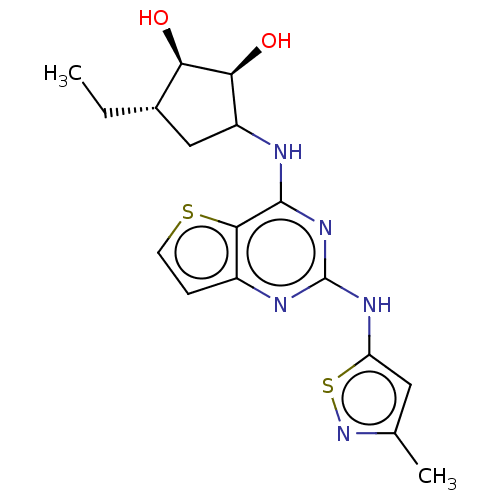 (US10988482, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING HANMI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAK4 kinase (purchased from Life Technologies, Cat. No.: PR5612U) was diluted to 2 folds of the final concentration (the final concentration is 0.76... | US Patent US10988482 (2021) BindingDB Entry DOI: 10.7270/Q29G5QZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 746 total ) | Next | Last >> |