

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 286.2 BDBM7459  Purchase Purchase | Wt: 270.2 BDBM7458  Purchase Purchase | Wt: 236.2 BDBM27660 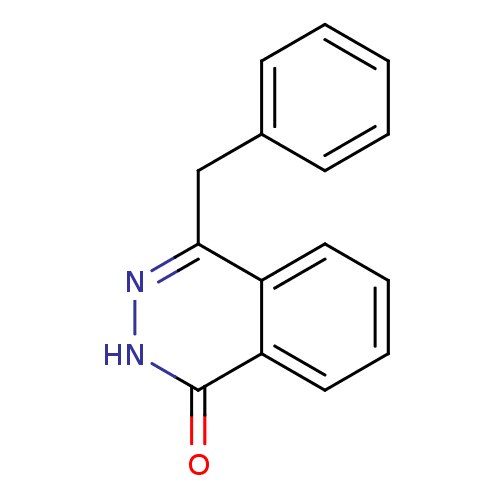 Purchase Purchase | Wt: 279.3 BDBM27703  Purchase Purchase | Wt: 194.6 BDBM27712 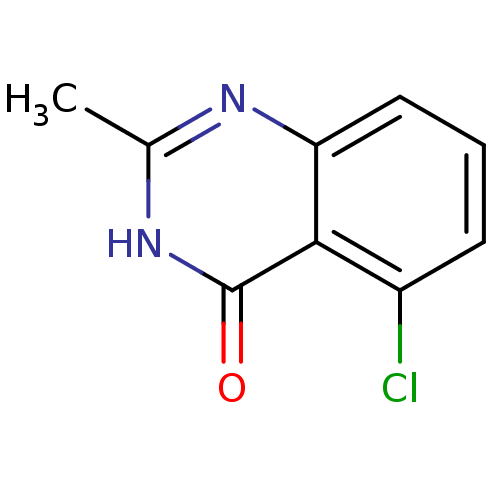 Purchase Purchase |
| Wt: 283.7 BDBM27720 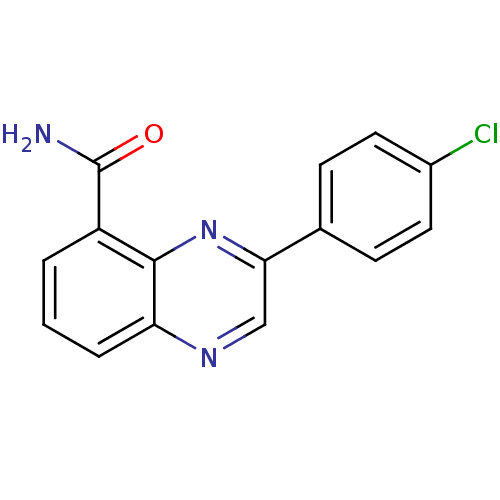 Purchase Purchase | Wt: 161.2 BDBM27682 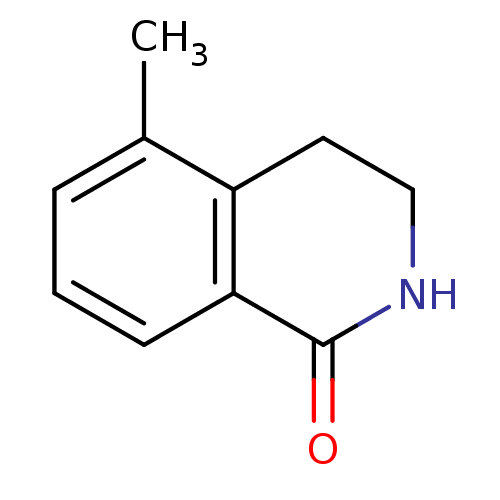 Purchase Purchase | Wt: 221.2 BDBM27686 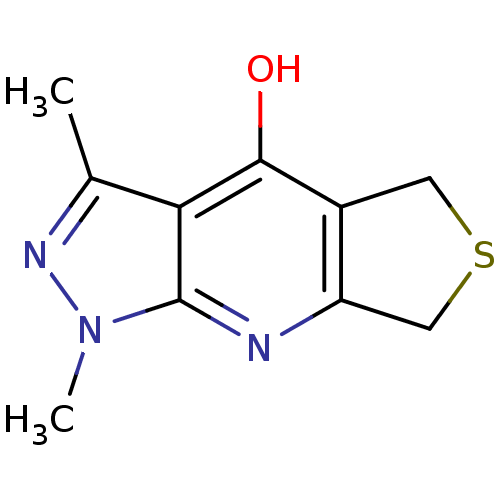 | Wt: 254.2 BDBM26659 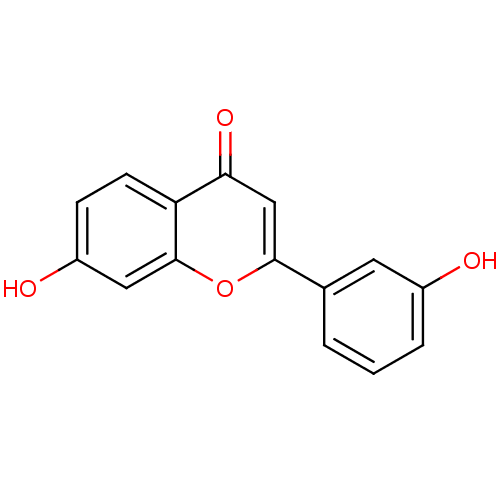 Purchase Purchase | Wt: 161.1 BDBM27088 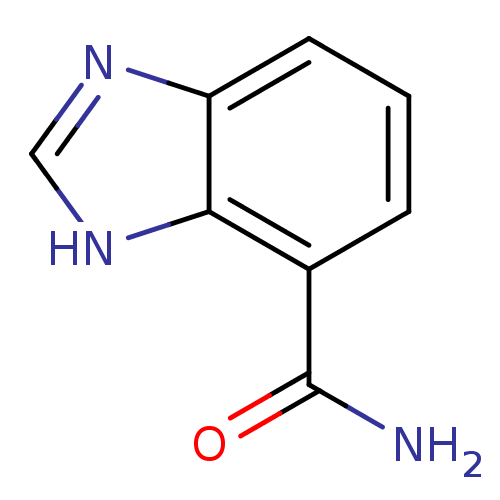 Purchase Purchase |
| Wt: 244.2 BDBM27135  Purchase Purchase | Wt: 244.2 BDBM27136 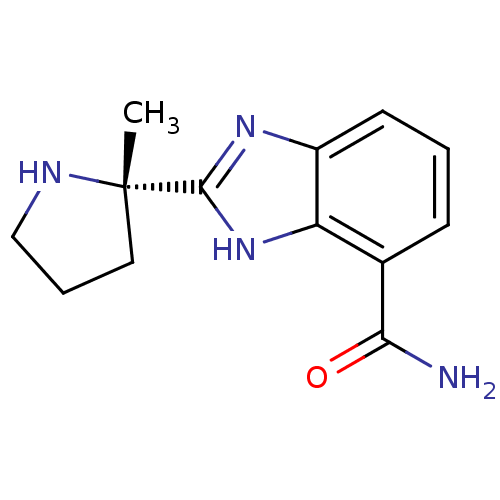 Purchase Purchase | Wt: 335.4 BDBM27493 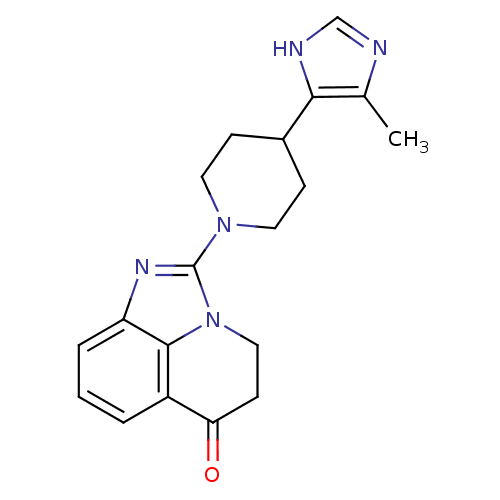 Purchase Purchase | Wt: 252.2 BDBM27496 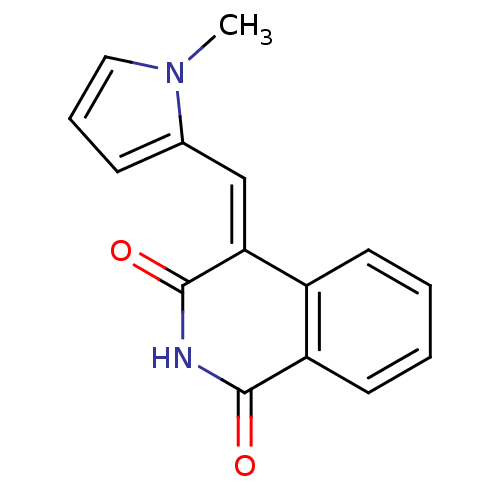 Purchase Purchase | Wt: 295.3 BDBM27497 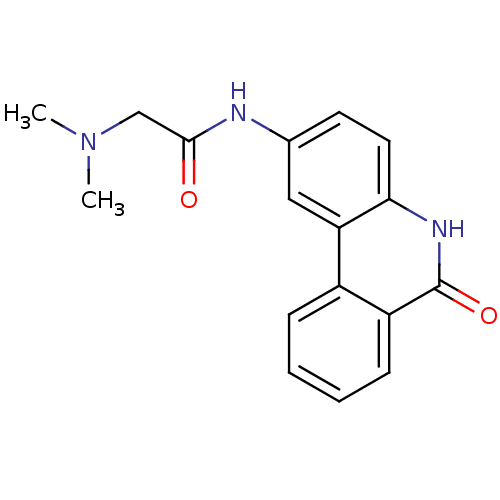 Purchase Purchase |
| Displayed 1 to 15 (of 171 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein mono-ADP-ribosyltransferase PARP3 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged PARP3 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinylat... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP2 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins foll... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP1 C-3-zinc finger domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 354 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP1 C-3-zinc finger domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged PARP2 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinylat... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged PARP2 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinylat... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein mono-ADP-ribosyltransferase PARP10 (Homo sapiens (Human)) | BDBM27497 (2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged PARP10 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinyla... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein mono-ADP-ribosyltransferase PARP10 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged PARP10 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinyla... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein mono-ADP-ribosyltransferase PARP10 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged PARP10 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinyla... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein mono-ADP-ribosyltransferase PARP14 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP14 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein mono-ADP-ribosyltransferase PARP15 (Homo sapiens (Human)) | BDBM27497 (2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP15 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein mono-ADP-ribosyltransferase PARP10 (Homo sapiens (Human)) | BDBM27497 (2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP10 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein mono-ADP-ribosyltransferase PARP10 (Homo sapiens (Human)) | BDBM27497 (2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP10 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins fol... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human TNKS2 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biot... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Health& Science University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged PARP1 ADP-ribosyltransferase domain expressed in Escherichia coli BL21(DE3) preincubated for 15 mins foll... | J Med Chem 60: 1262-1271 (2017) Article DOI: 10.1021/acs.jmedchem.6b00990 BindingDB Entry DOI: 10.7270/Q28054VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase assessed as reduction in uric acid formation using xanthine as substrate pretreated for 5 mins followed by subs... | Eur J Med Chem 131: 14-28 (2017) Article DOI: 10.1016/j.ejmech.2017.03.002 BindingDB Entry DOI: 10.7270/Q2W95CGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoperoxidase (Bos taurus (Bovine)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of bovine milk LPO assessed as reduction in NaOSCN production in presence of H2O2/NaSCN after 5 mins | J Med Chem 60: 6563-6586 (2017) Article DOI: 10.1021/acs.jmedchem.7b00285 BindingDB Entry DOI: 10.7270/Q27D2XD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of recombinant MPO (unknown origin) assessed as reduction in taurine chloramine production preincubated with enzyme and taurine followed b... | J Med Chem 60: 6563-6586 (2017) Article DOI: 10.1021/acs.jmedchem.7b00285 BindingDB Entry DOI: 10.7270/Q27D2XD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 653 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The FP assay was then adapted for HTS and used to screen a ˜120,000 member small molecule library for compounds that displaced the FP probe from the ... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2H70J43 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Faculty of Medicine, University of Ni?, Bulevar Dr Zorana?in?i?a 81, 18000 Ni?, Serbia. Curated by ChEMBL | Assay Description Inhibition of bovine milk xanthine oxidase assessed using xanthine as substrate after 10 mins by fluorometric assay | Eur J Med Chem 135: 491-516 (2017) Article DOI: 10.1016/j.ejmech.2017.04.031 BindingDB Entry DOI: 10.7270/Q2VD71XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Faculty of Medicine, University of Ni?, Bulevar Dr Zorana?in?i?a 81, 18000 Ni?, Serbia. Curated by ChEMBL | Assay Description Inhibition of bovine milk xanthine oxidase assessed as reduction in uric acid formation using xanthine as substrate preincubated for 3 hrs followed b... | Eur J Med Chem 135: 491-516 (2017) Article DOI: 10.1016/j.ejmech.2017.04.031 BindingDB Entry DOI: 10.7270/Q2VD71XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using kynuramine as substrate incubated for 20 mins by spectrophotometric method | Bioorg Med Chem Lett 28: 2403-2407 (2018) Article DOI: 10.1016/j.bmcl.2018.06.023 BindingDB Entry DOI: 10.7270/Q23R0WCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP1 expressed in Escherichia coli BL21(DE3) using sheared DNA as substrate measured after 1 hr in presence of NAD+ | Eur J Med Chem 132: 26-41 (2017) Article DOI: 10.1016/j.ejmech.2017.03.013 BindingDB Entry DOI: 10.7270/Q2S184XW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP1 expressed in Escherichia coli BL21(DE3) using histone as substrate measured after 1 hr in presence of biotinyla... | Eur J Med Chem 132: 26-41 (2017) Article DOI: 10.1016/j.ejmech.2017.03.013 BindingDB Entry DOI: 10.7270/Q2S184XW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in supersomes using ethoxyresorufin as substrate preincubated for 5 mins followed by substrate addit... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in supersomes using ethoxyresorufin as substrate preincubated for 5 mins followed by substrate addit... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A assessed as reduction in 4-hydroxyquinoline formation using kynuramine as substrate after 20 mins by fluorometr... | J Nat Prod 79: 2538-2544 (2016) Article DOI: 10.1021/acs.jnatprod.6b00440 BindingDB Entry DOI: 10.7270/Q26W9DHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B assessed as reduction in 4-hydroxyquinoline formation using kynuramine as substrate after 20 mins by fluorometr... | J Nat Prod 79: 2538-2544 (2016) Article DOI: 10.1021/acs.jnatprod.6b00440 BindingDB Entry DOI: 10.7270/Q26W9DHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myocilin (Homo sapiens) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 4.16E+4 | n/a | n/a | n/a | n/a | n/a |
Center for the Study of Systems Biology, School of Biology, Georgia Institute of Technology, 950, Atlantic Drive, Atlanta, GA 30332, United States. Curated by ChEMBL | Assay Description Binding affinity myocilin-OLF domain (unknown origin) by SRP assay | Bioorg Med Chem Lett 27: 4133-4139 (2017) Article DOI: 10.1016/j.bmcl.2017.07.035 BindingDB Entry DOI: 10.7270/Q2NG4T4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human 17beta-HSD2 expressed in HEK293 cell lysates incubated for 10 mins using [2,4,6,7-3H]-estradiol and NAD+ by scintillation countin... | J Nat Prod 80: 965-974 (2017) Article DOI: 10.1021/acs.jnatprod.6b00950 BindingDB Entry DOI: 10.7270/Q2XD148R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using kynuramine as substrate preincubated for 30 mins followed by substrate addition | Bioorg Med Chem Lett 28: 584-588 (2018) Article DOI: 10.1016/j.bmcl.2018.01.049 BindingDB Entry DOI: 10.7270/Q25T3P2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 2 (Homo sapiens (Human)) | BDBM27497 (2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Center for Biomedical REsearch Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition against 4-hydroxyphenylpyruvate dioxygenase from pig liver; (observed value) | J Nat Prod 80: 2741-2750 (2017) Article DOI: 10.1021/acs.jnatprod.7b00515 BindingDB Entry DOI: 10.7270/Q2GH9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of NAD+ by ELISA | Eur J Med Chem 145: 389-403 (2018) Article DOI: 10.1016/j.ejmech.2018.01.018 BindingDB Entry DOI: 10.7270/Q2PG1VDR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human PARP2 using histone as substrate after 1.5 hr in presence of NAD+ by ELISA | Eur J Med Chem 145: 389-403 (2018) Article DOI: 10.1016/j.ejmech.2018.01.018 BindingDB Entry DOI: 10.7270/Q2PG1VDR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histidine kinase (Streptococcus pneumoniae PCS8203) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae VicK preincubated for 30 mins followed by [gamma-33P]ATP addition after 30 mins by SDS-PAGE method | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chemotaxis protein CheA (Escherichia coli (strain K12)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of Escherichia coli CheA preincubated for 30 mins followed by [gamma-33P]ATP addition after 30 mins by SDS-PAGE method | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... | Eur J Med Chem 151: 145-157 (2018) Article DOI: 10.1016/j.ejmech.2018.03.041 BindingDB Entry DOI: 10.7270/Q2CZ39SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... | Eur J Med Chem 151: 145-157 (2018) Article DOI: 10.1016/j.ejmech.2018.03.041 BindingDB Entry DOI: 10.7270/Q2CZ39SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Ovis aries) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]-estradiol (E2) from sheep uterine estrogen receptor | J Med Chem 47: 1018-30 (2004) Article DOI: 10.1021/jm0309607 BindingDB Entry DOI: 10.7270/Q2VM4G1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Binding affinity for estrogen receptor, by competition with [3H]estradiol | J Med Chem 39: 3461-9 (1996) Article DOI: 10.1021/jm950938g BindingDB Entry DOI: 10.7270/Q2ZP48V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homeobox protein Nkx-2.5/Transcription factor GATA-4 (Mus musculus) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki , Helsinki FI-00014, Finland. Curated by ChEMBL | Assay Description Inhibition of mouse GATA4/NKX2-5 transcriptional synergy expressed in African green monkey COS-1 cells measured after 30 hrs by dual luciferase repor... | J Med Chem 60: 7781-7798 (2017) Article DOI: 10.1021/acs.jmedchem.7b00816 BindingDB Entry DOI: 10.7270/Q2DN47GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homeobox protein Nkx-2.5/Transcription factor GATA-4 (Mus musculus) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki , Helsinki FI-00014, Finland. Curated by ChEMBL | Assay Description Inhibition of mouse GATA4/NKX2-5 transcriptional synergy expressed in African green monkey COS-1 cells measured after 30 hrs by dual luciferase repor... | J Med Chem 60: 7781-7798 (2017) Article DOI: 10.1021/acs.jmedchem.7b00816 BindingDB Entry DOI: 10.7270/Q2DN47GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanine--D-alanine ligase (Helicobacter pylori (strain HPAG1)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Helicobacter pylori Ddl using ATP as substrate | Citation and Details Article DOI: 10.1007/s00044-012-0207-7 BindingDB Entry DOI: 10.7270/Q2CZ3B2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanine--D-alanine ligase B (Escherichia coli K-12) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Escherichia coli DdlB using ATP as substrate | Citation and Details Article DOI: 10.1007/s00044-012-0207-7 BindingDB Entry DOI: 10.7270/Q2CZ3B2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant amyloid beta (1 to 42) fibrils (unknown origin) by thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using daunorubicin as substrate incubated for... | J Nat Prod 78: 2666-74 (2015) Article DOI: 10.1021/acs.jnatprod.5b00616 BindingDB Entry DOI: 10.7270/Q2XG9V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged AKR1B1 (unknown origin) expressed in Escherichia coli BL21 cells using all-trans-retinal as substrat... | J Nat Prod 78: 2666-74 (2015) Article DOI: 10.1021/acs.jnatprod.5b00616 BindingDB Entry DOI: 10.7270/Q2XG9V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| << First | Previous | Displayed 301 to 350 (of 466 total ) | Next | Last >> |
| Cell (A) | Syringe (B) | Cell Links | Syringe Links | Cell + Syr Links | ΔG° kcal/mole | -TΔS° kcal/mole | ΔH° kcal/mole | log K | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|
| Transcriptional Regulator TtgR (Pseudomonas putida) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | GoogleScholar PDB | CHEBI KEGG MMDB PC cid PC sid PDB | -7.12 | -4.08 | -3.05 | 5.14 | 7 | 30 | |
Estacion Experimental del Zaidin | J Biol Chem 281: 7102-9 (2006) | |||||||||
| Transcriptional Regulator TtgR (Pseudomonas putida) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | GoogleScholar PDB | CHEBI DrugBank KEGG MMDB PC cid PC sid PDB | -6.66 | -5.38 | -1.28 | 4.81 | 7 | 30 | |
Estacion Experimental del Zaidin | J Biol Chem 281: 7102-9 (2006) | |||||||||