 Found 2221 hits for monomerid = 8961,17638,21278,21279,21281,21282,21283,21284,21311,20461,20462,22952,22988,24567,26739
Found 2221 hits for monomerid = 8961,17638,21278,21279,21281,21282,21283,21284,21311,20461,20462,22952,22988,24567,26739 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 420-5 (2001)
BindingDB Entry DOI: 10.7270/Q2VH5MCT |
More data for this
Ligand-Target Pair | |
Choline O-acetyltransferase
(RAT) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition constant determined against Acetylcholinesterase (AChE) receptor. |
J Med Chem 41: 4186-9 (1998)
Checked by Author
Article DOI: 10.1021/jm9810452
BindingDB Entry DOI: 10.7270/Q2HH6KRM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21284
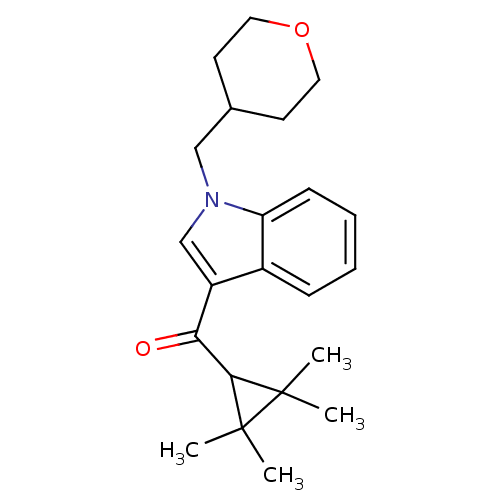
(1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...)Show SMILES CC1(C)C(C(=O)c2cn(CC3CCOCC3)c3ccccc23)C1(C)C Show InChI InChI=1S/C22H29NO2/c1-21(2)20(22(21,3)4)19(24)17-14-23(13-15-9-11-25-12-10-15)18-8-6-5-7-16(17)18/h5-8,14-15,20H,9-13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... |
J Med Chem 51: 1904-12 (2008)
Article DOI: 10.1021/jm7011613
BindingDB Entry DOI: 10.7270/Q2C827K2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21284

(1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...)Show SMILES CC1(C)C(C(=O)c2cn(CC3CCOCC3)c3ccccc23)C1(C)C Show InChI InChI=1S/C22H29NO2/c1-21(2)20(22(21,3)4)19(24)17-14-23(13-15-9-11-25-12-10-15)18-8-6-5-7-16(17)18/h5-8,14-15,20H,9-13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
J Med Chem 53: 295-315 (2010)
Article DOI: 10.1021/jm901214q
BindingDB Entry DOI: 10.7270/Q2KD1Z00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pontificia Universidad Cat£lica de Chile
Curated by ChEMBL
| Assay Description
Agonist activity at CB2 receptor (unknown origin) |
Eur J Med Chem 124: 17-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.005
BindingDB Entry DOI: 10.7270/Q2Q2426V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Binding affinity to displace [3H]CP-55,940 from cloned human CB2 receptor |
Bioorg Med Chem Lett 15: 4110-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.008
BindingDB Entry DOI: 10.7270/Q2KD1XGV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by filtration assay |
Bioorg Med Chem 20: 2067-81 (2012)
Article DOI: 10.1016/j.bmc.2012.01.038
BindingDB Entry DOI: 10.7270/Q2PG1SW6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 420-5 (2001)
BindingDB Entry DOI: 10.7270/Q2VH5MCT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity of compound was determined against to human cannabinoid receptor 2 in chinese hamster ovary cells |
J Med Chem 46: 2110-6 (2003)
Article DOI: 10.1021/jm020329q
BindingDB Entry DOI: 10.7270/Q2J67HPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 2019-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.025
BindingDB Entry DOI: 10.7270/Q2WS8VM2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Cell Chem Biol 56: 8224-56 (2013)
Article DOI: 10.1021/jm4005626
BindingDB Entry DOI: 10.7270/Q2B859M5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 359-68 (2002)
Article DOI: 10.1124/jpet.302.1.359
BindingDB Entry DOI: 10.7270/Q2DZ06VP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 420-5 (2001)
BindingDB Entry DOI: 10.7270/Q2VH5MCT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in CHO-K1 cells by liquid scintillation counting |
Eur J Med Chem 85: 77-86 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.062
BindingDB Entry DOI: 10.7270/Q2NS0WKT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | -13.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bern , Bühlstrasse 28, CH-3012 Bern, Switzerland.
| Assay Description
In this assay, 18 ug of crude membrane expressing hCB1 or hCB2 receptors were re-suspended in 300 uL of binding buffer containing 50 mM Tris-HCl, 2.5... |
ACS Chem Biol 9: 1499-507 (2014)
Article DOI: 10.1021/cb500177c
BindingDB Entry DOI: 10.7270/Q2T15295 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(MOUSE) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 420-5 (2001)
BindingDB Entry DOI: 10.7270/Q2VH5MCT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21279
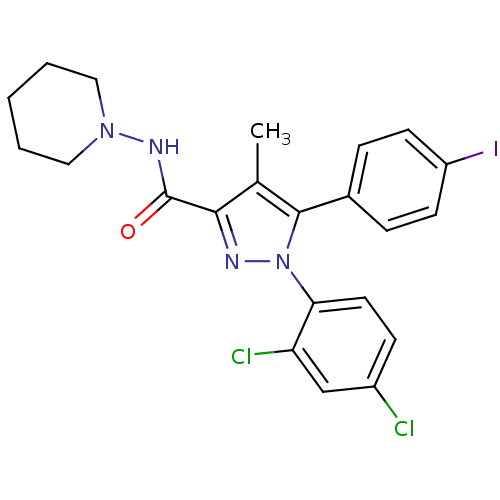
(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 |
J Med Chem 46: 642-5 (2003)
Article DOI: 10.1021/jm020157x
BindingDB Entry DOI: 10.7270/Q27M079X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | -12.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... |
J Med Chem 52: 234-7 (2009)
Article DOI: 10.1021/jm8012932
BindingDB Entry DOI: 10.7270/Q2CN7276 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO cell membranes after 1 hr by liquid scintillation spectrometry |
Bioorg Med Chem 26: 4518-4531 (2018)
Article DOI: 10.1016/j.bmc.2018.07.043
BindingDB Entry DOI: 10.7270/Q2VM4FW4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-725519 from human CB1 receptor expressed in CHO cells after 90 mins by scintillation counting |
Bioorg Med Chem Lett 21: 6856-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.016
BindingDB Entry DOI: 10.7270/Q2WS8TN3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells after 3 hrs by liquid scintillation counting |
Eur J Med Chem 45: 1133-9 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.018
BindingDB Entry DOI: 10.7270/Q2K937P0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from rat brain CB1 receptor |
Bioorg Med Chem Lett 19: 5351-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.130
BindingDB Entry DOI: 10.7270/Q2KP827S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | -12.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... |
J Med Chem 52: 234-7 (2009)
Article DOI: 10.1021/jm8012932
BindingDB Entry DOI: 10.7270/Q2CN7276 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2034-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.019
BindingDB Entry DOI: 10.7270/Q27082PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories
Curated by ChEMBL
| Assay Description
Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay |
Bioorg Med Chem Lett 16: 731-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.019
BindingDB Entry DOI: 10.7270/Q2TM79PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corbus Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in HEK293T cell membranes by scintillation counting |
Bioorg Med Chem 22: 3245-51 (2014)
Article DOI: 10.1016/j.bmc.2014.04.062
BindingDB Entry DOI: 10.7270/Q22Z173H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 2706-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.011
BindingDB Entry DOI: 10.7270/Q2H70FGB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in CHO-K1 cells |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation |
J Med Chem 45: 2708-19 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3DQW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 292: 886-94 (2000)
BindingDB Entry DOI: 10.7270/Q2542M4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant cannabinoid CB2 receptor expressed in HEK293 cells |
Bioorg Med Chem 17: 2842-51 (2009)
Article DOI: 10.1016/j.bmc.2009.02.027
BindingDB Entry DOI: 10.7270/Q23R0TSC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1
(Mus musculus (mouse)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... |
J Med Chem 55: 6898-915 (2012)
Article DOI: 10.1021/jm300689c
BindingDB Entry DOI: 10.7270/Q2ZK5HSK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum |
J Med Chem 49: 872-82 (2006)
Article DOI: 10.1021/jm050484f
BindingDB Entry DOI: 10.7270/Q28916NK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | 125 | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... |
J Med Chem 51: 1904-12 (2008)
Article DOI: 10.1021/jm7011613
BindingDB Entry DOI: 10.7270/Q2C827K2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 420-5 (2001)
BindingDB Entry DOI: 10.7270/Q2VH5MCT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-CP-55,940 binding to cannabinoid receptor 1 in rat brain membranes. |
J Med Chem 45: 1447-59 (2002)
BindingDB Entry DOI: 10.7270/Q2222T3Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55940 from human CB2 receptor in cell free system |
Eur J Med Chem 46: 547-55 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.034
BindingDB Entry DOI: 10.7270/Q2CF9QCJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
J Med Chem 53: 295-315 (2010)
Article DOI: 10.1021/jm901214q
BindingDB Entry DOI: 10.7270/Q2KD1Z00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor |
J Med Chem 54: 2961-70 (2011)
Article DOI: 10.1021/jm2000536
BindingDB Entry DOI: 10.7270/Q2KS6SJS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain membrane without cerebellum by liquid scintillation counting analysis |
Eur J Med Chem 62: 256-69 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.056
BindingDB Entry DOI: 10.7270/Q2TQ62W2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay |
Bioorg Med Chem Lett 19: 5351-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.130
BindingDB Entry DOI: 10.7270/Q2KP827S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase