 Found 14 hits in this display
Found 14 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polymerase acidic protein
(Hepatitis C virus) | BDBM33410
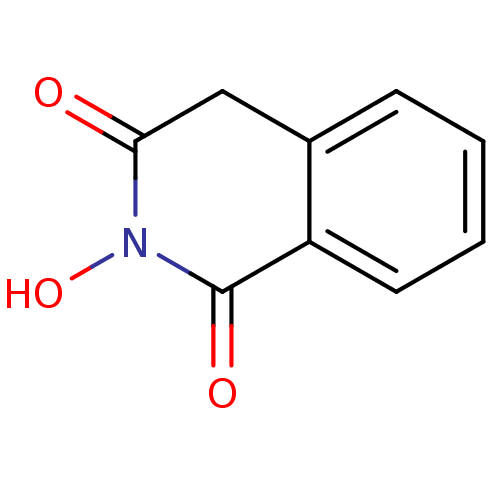
(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity to PA N-terminal domain (Competitive) |
ACS Chem Biol 7: 526-34 (2012)
Article DOI: 10.1021/cb200439z
BindingDB Entry DOI: 10.7270/Q2JQ121G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc.
| Assay Description
Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... |
J Med Chem 52: 5781-4 (2009)
Article DOI: 10.1021/jm900597q
BindingDB Entry DOI: 10.7270/Q2TM78F2 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of reconstituted HIV1 RNase H using RNA/DNA duplex substrate by fluorescence assay |
J Med Chem 58: 651-64 (2015)
Article DOI: 10.1021/jm501132s
BindingDB Entry DOI: 10.7270/Q2W95BWG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in RNA 5' end directed clea... |
J Med Chem 58: 651-64 (2015)
Article DOI: 10.1021/jm501132s
BindingDB Entry DOI: 10.7270/Q2W95BWG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in DNA 3' end directed clea... |
J Med Chem 58: 651-64 (2015)
Article DOI: 10.1021/jm501132s
BindingDB Entry DOI: 10.7270/Q2W95BWG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in internal cleavage using ... |
J Med Chem 58: 651-64 (2015)
Article DOI: 10.1021/jm501132s
BindingDB Entry DOI: 10.7270/Q2W95BWG |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 1
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase |
J Med Chem 51: 7717-30 (2008)
Article DOI: 10.1021/jm8007085
BindingDB Entry DOI: 10.7270/Q2222XJ1 |
More data for this
Ligand-Target Pair | |
Polymerase acidic protein
(Hepatitis C virus) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cap-dependent endonuclease activity of influenza virus RNP |
J Med Chem 46: 1153-64 (2003)
Article DOI: 10.1021/jm020334u
BindingDB Entry DOI: 10.7270/Q22J6B6V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polymerase acidic protein
(Hepatitis C virus) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A virus (A/PR/8/34) PA endonuclease using ALMV cap1 primer RNA as substrate after 90 mins by scintillation counting |
J Med Chem 60: 3533-3551 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01227
BindingDB Entry DOI: 10.7270/Q2VQ34XH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant reverse transcriptase polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 mins by li... |
J Med Chem 58: 651-64 (2015)
Article DOI: 10.1021/jm501132s
BindingDB Entry DOI: 10.7270/Q2W95BWG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 1
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 51: 7717-30 (2008)
Article DOI: 10.1021/jm8007085
BindingDB Entry DOI: 10.7270/Q2222XJ1 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
Eur J Med Chem 83: 609-16 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.061
BindingDB Entry DOI: 10.7270/Q2MG7R34 |
More data for this
Ligand-Target Pair | |
Polymerase acidic protein
(Hepatitis C virus) | BDBM33410

(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Plaque growth inhibition |
ACS Chem Biol 7: 526-34 (2012)
Article DOI: 10.1021/cb200439z
BindingDB Entry DOI: 10.7270/Q2JQ121G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |