

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50027783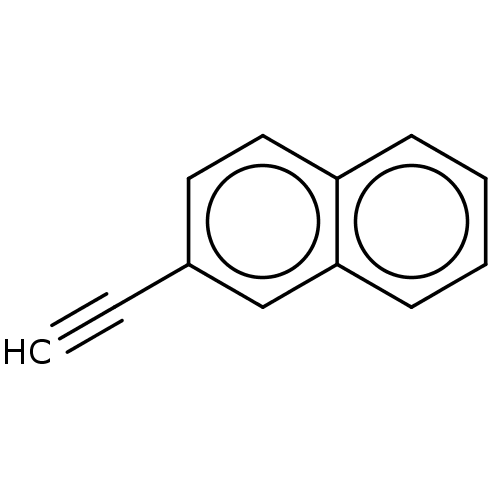 (2-Ethynyl Naphtalene | CHEMBL1743361) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-ethoxycoumarin O-deethylase activity | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50027784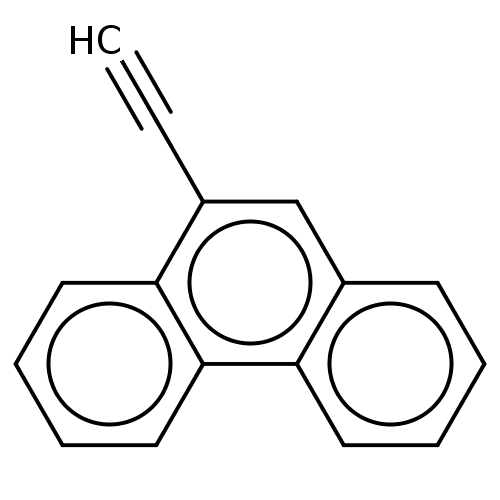 (9-Ethynyl Phenanthrene | CHEMBL1908227) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-pentoxyresorufin O-deethylation activity (PROD) | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B11 (Canis familiaris) | BDBM50027786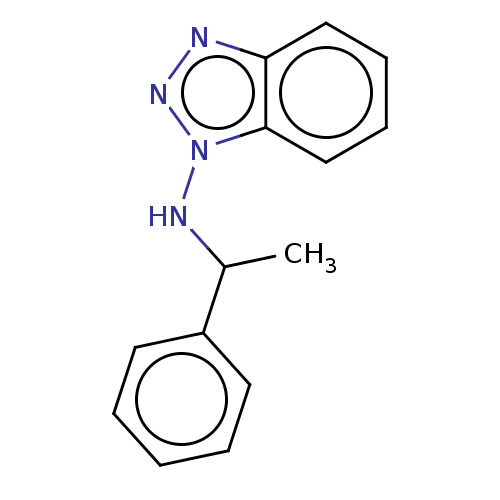 (CHEMBL1908236) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of dog cytochrome P450 CYP2B11 measured by Diazepam N1-demethylation using recombinant enzyme | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B11 (Canis familiaris) | BDBM50027786 (CHEMBL1908236) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of dog cytochrome P450 CYP2B11 measured by Diazepam N1-demethylation using liver microsomes | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50003140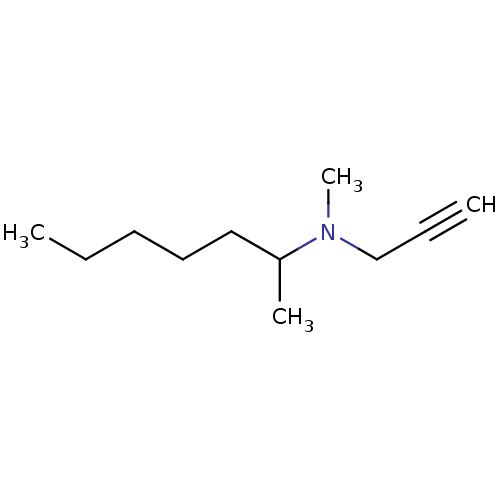 (CHEMBL541325 | Methyl-(1-methyl-hexyl)-prop-2-ynyl...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-pentoxyresorufin O-deethylation activity (PROD) | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-EFC O-deethylation activity | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-EFC O-deethylation activity | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50310823 (CHEMBL1078442 | bergamottin) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 2B1 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50027787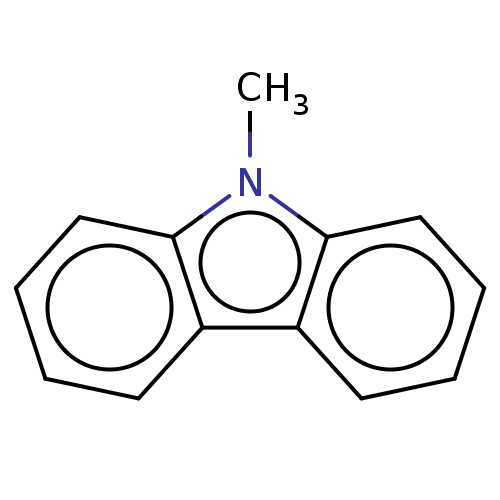 (CHEMBL1908211 | N-Methylcarbazole) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 CYP2B1 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50240520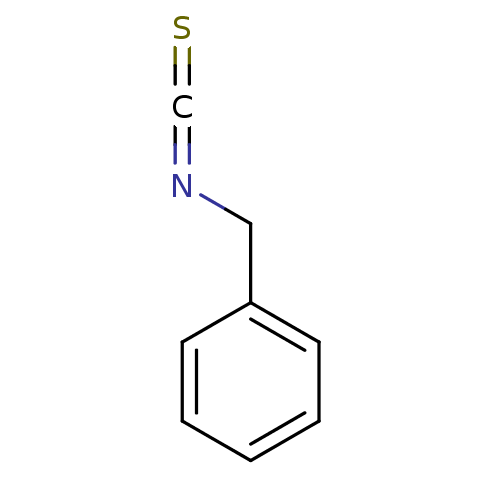 ((isothiocyanatomethyl)benzene | BITC, 17 | Benzyls...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 CYP2B1 measured by 7-EFC O-deethylation activity | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50187243 (17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 2B1 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50418085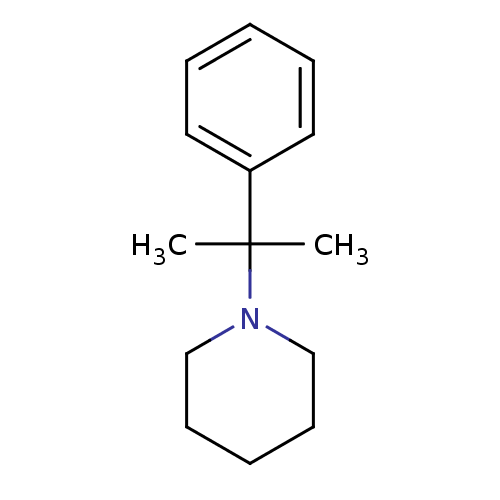 (CHEMBL1743344) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 2B1 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50418086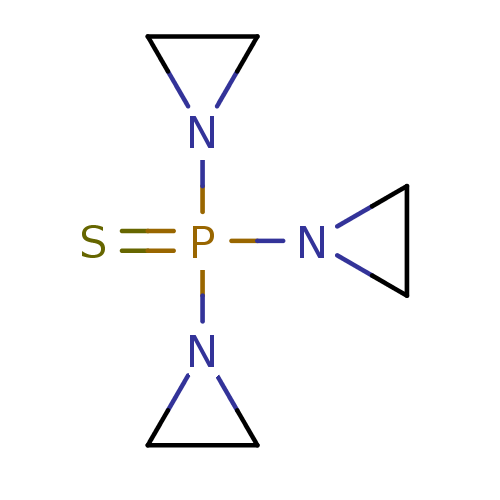 (THIOTEPA | Thioplex) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of rat cytochrome P450 2B1 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM24656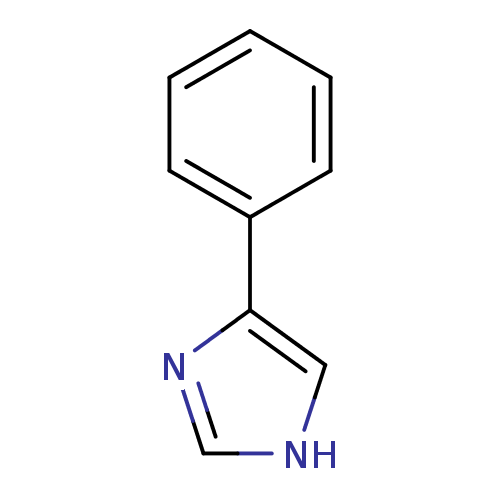 (4-PIM | 4-Phenylimidazole | 4-phenyl-1H-imidazole ...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50017716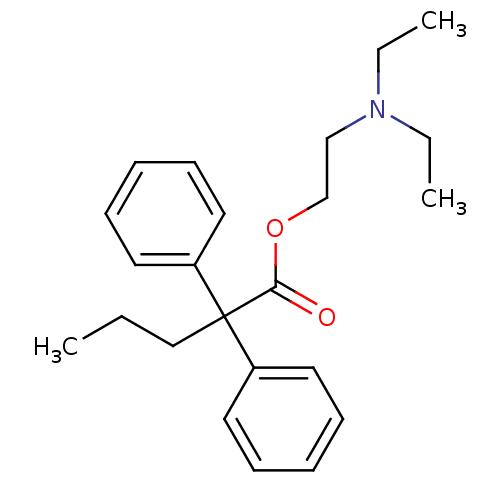 (2,2-Diphenyl-pentanoic acid 2-diethylamino-ethyl e...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404886 (CHEMBL169709) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404883 (CHEMBL169858) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404941 (CHEMBL353984) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404855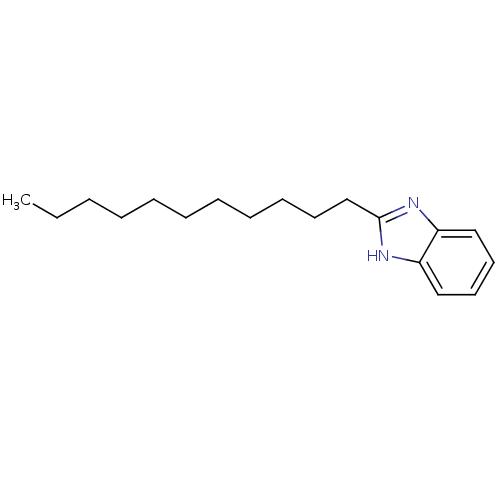 (CHEMBL158007) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404893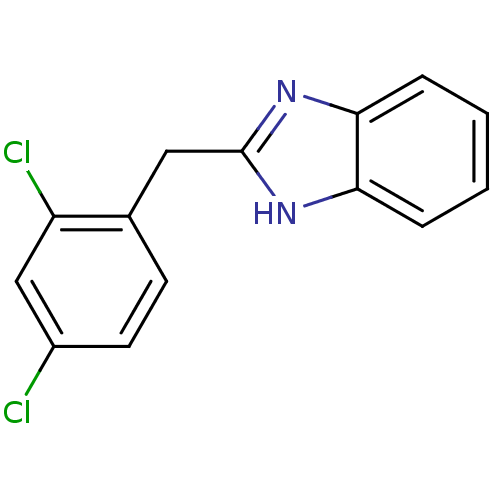 (CHEMBL355666) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404855 (CHEMBL158007) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404888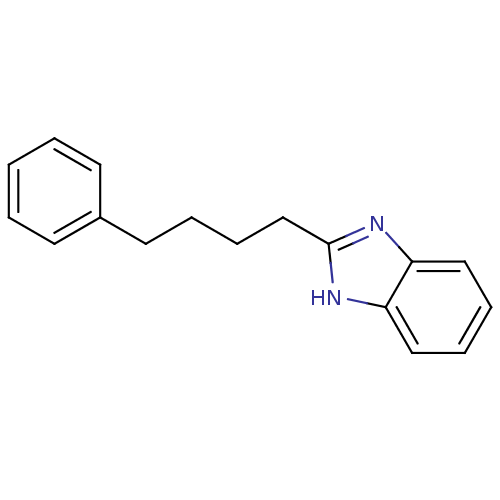 (CHEMBL169203) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404902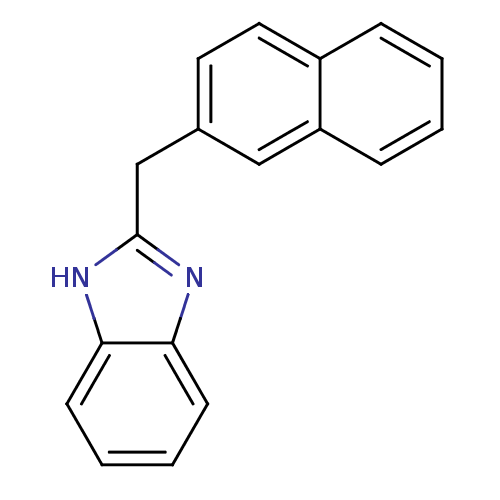 (CHEMBL355669) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404903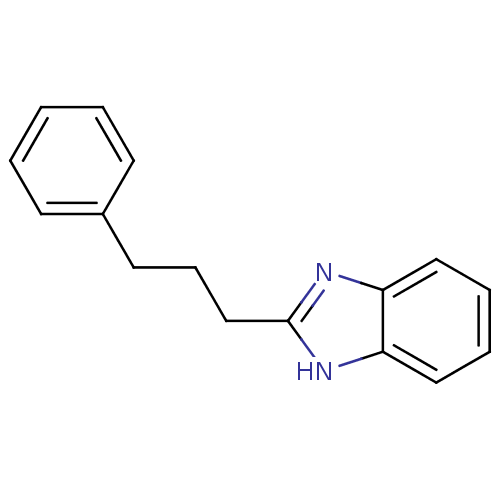 (CHEMBL172621) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM39194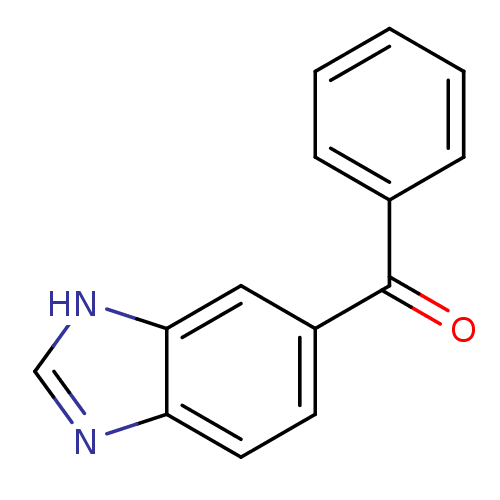 ((1H-Benzoimidazol-5-yl)-phenyl-methanone | 3H-benz...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404916 (CHEMBL171325) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404936 (CHEMBL169212) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404925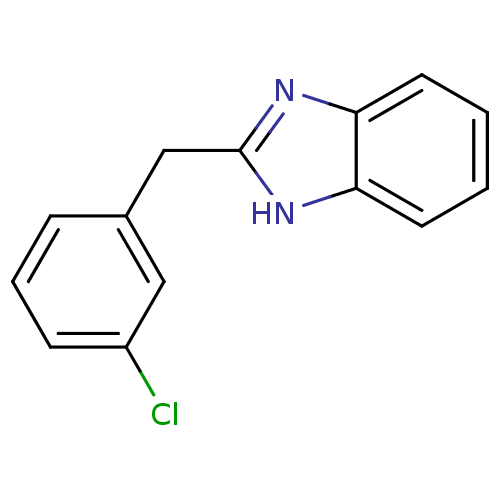 (CHEMBL354972) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404905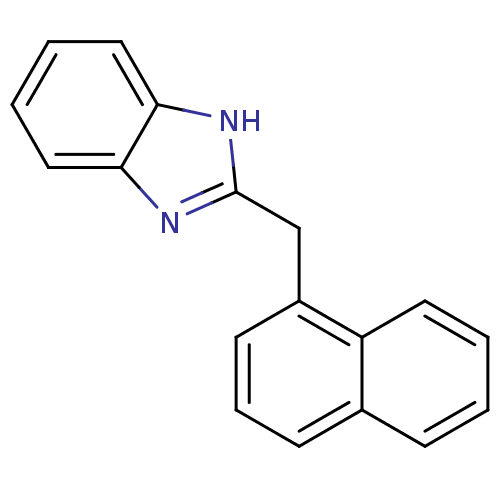 (CHEMBL170513) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404930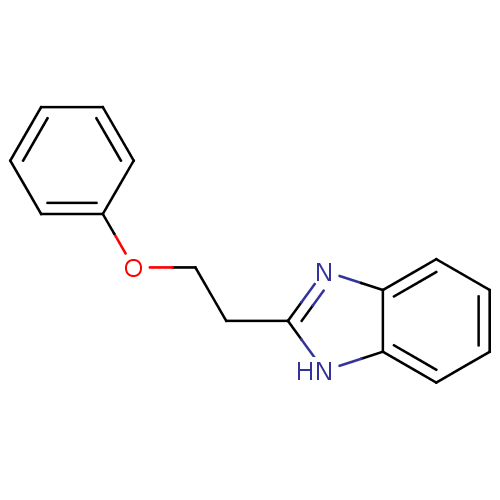 (CHEMBL170998) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404896 (CHEMBL355280) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404852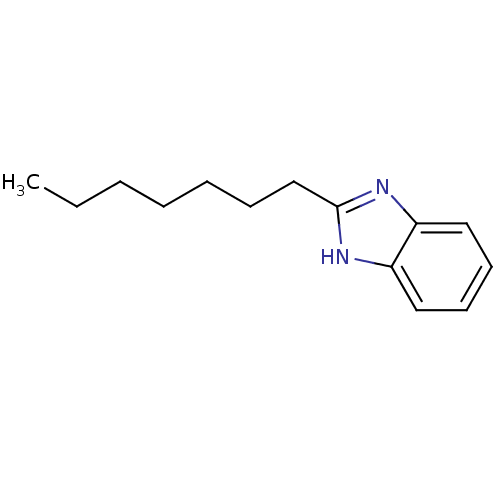 (CHEMBL345675) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404942 (CHEMBL353595) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404944 (CHEMBL424396) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404852 (CHEMBL345675) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404907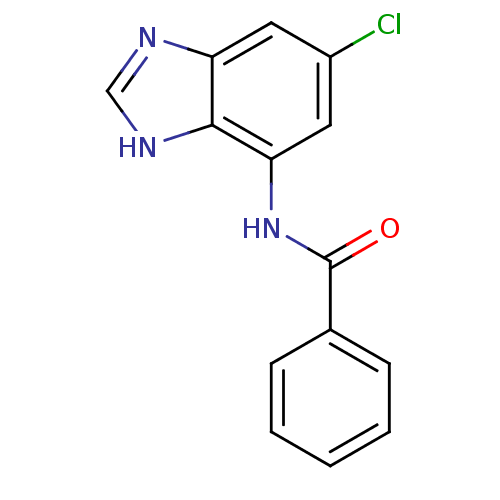 (CHEMBL172114) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404934 (CHEMBL172156) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404931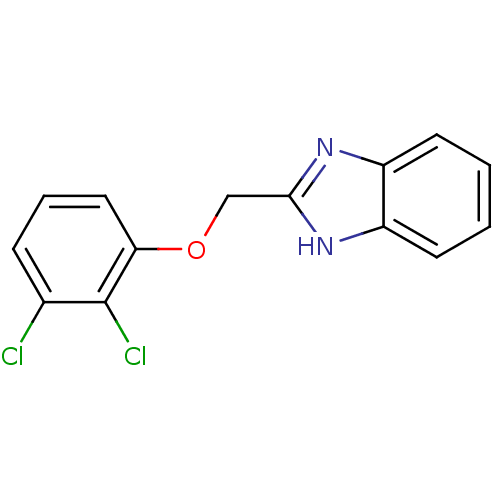 (CHEMBL354514) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404938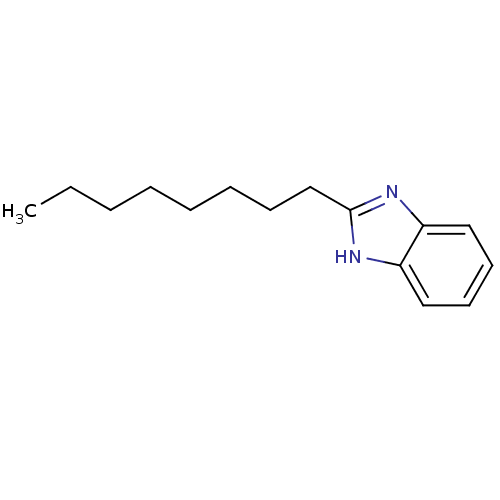 (CHEMBL170771) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404932 (CHEMBL169149) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404909 (CHEMBL352972) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404891 (CHEMBL353945) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404921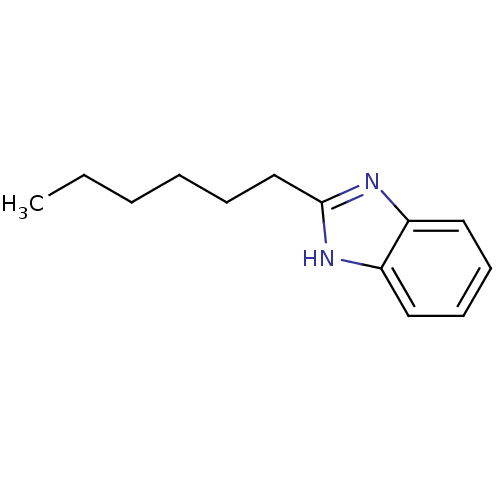 (CHEMBL171191) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50240909 (2-Phenethyl-1H-benzoimidazole | 2-phenethyl-1H-ben...) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404904 (CHEMBL170910) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404940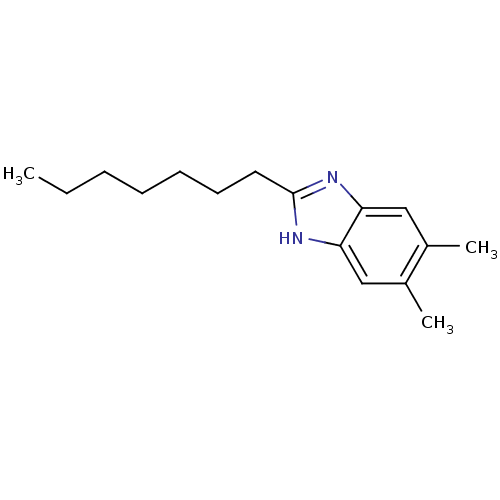 (CHEMBL169701) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404926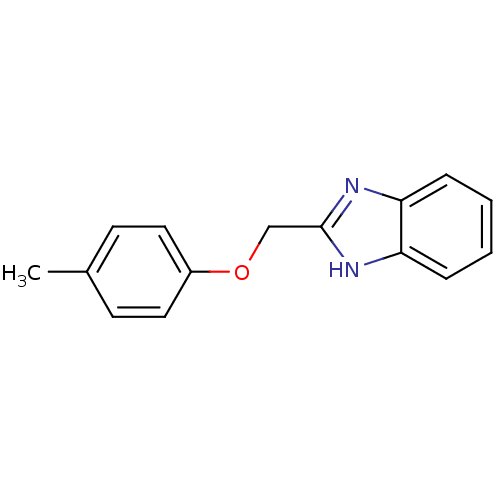 (CHEMBL355159) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404854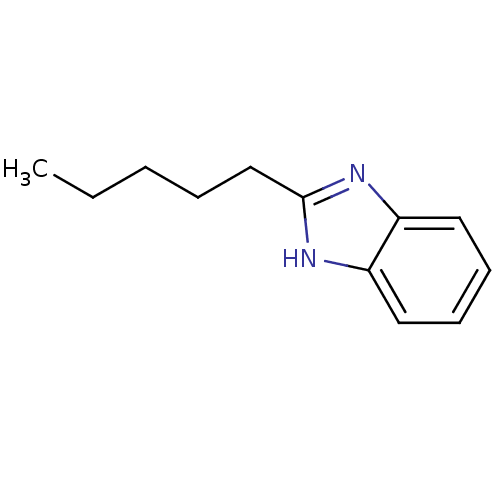 (CHEMBL155867) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404854 (CHEMBL155867) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404853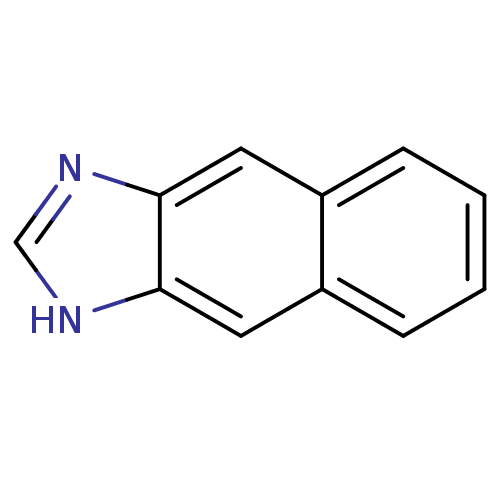 (CHEMBL156313) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |