 Found 29 hits Enz. Inhib. hit(s) with Target = 'DNA topoisomerase 4 subunit B'
Found 29 hits Enz. Inhib. hit(s) with Target = 'DNA topoisomerase 4 subunit B' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50393079
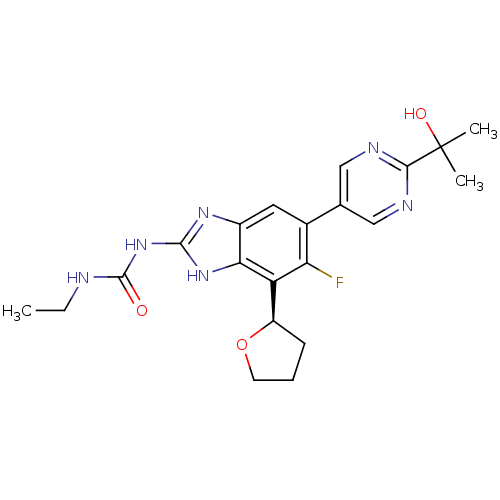
(CHEMBL2152855 | US9040542, 23)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)O |r| Show InChI InChI=1S/C21H25FN6O3/c1-4-23-20(29)28-19-26-13-8-12(11-9-24-18(25-10-11)21(2,3)30)16(22)15(17(13)27-19)14-6-5-7-31-14/h8-10,14,30H,4-7H2,1-3H3,(H3,23,26,27,28,29)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA topoisomerase 4 Par E subunit by ATPase assay |
ACS Med Chem Lett 3: 783-784 (2012)
Article DOI: 10.1021/ml300234y
BindingDB Entry DOI: 10.7270/Q2N29Z1N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50393080
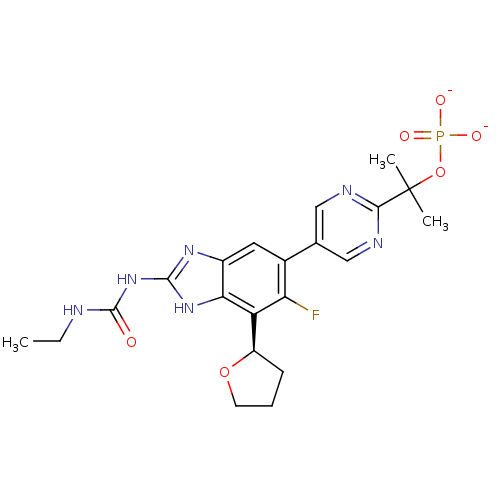
(CHEMBL2152856)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)OP([O-])([O-])=O |r| Show InChI InChI=1S/C21H26FN6O6P/c1-4-23-20(29)28-19-26-13-8-12(16(22)15(17(13)27-19)14-6-5-7-33-14)11-9-24-18(25-10-11)21(2,3)34-35(30,31)32/h8-10,14H,4-7H2,1-3H3,(H2,30,31,32)(H3,23,26,27,28,29)/p-2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA topoisomerase 4 Par E subunit by ATPase assay |
ACS Med Chem Lett 3: 783-784 (2012)
Article DOI: 10.1021/ml300234y
BindingDB Entry DOI: 10.7270/Q2N29Z1N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470392
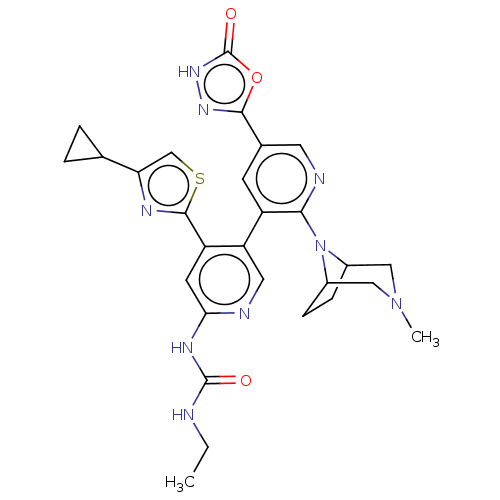
(CHEMBL4283208)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C2CC2)c(cn1)-c1cc(cnc1N1C2CCC1CN(C)C2)-c1n[nH]c(=O)o1 Show InChI InChI=1S/C28H31N9O3S/c1-3-29-27(38)33-23-9-20(26-32-22(14-41-26)15-4-5-15)21(11-30-23)19-8-16(25-34-35-28(39)40-25)10-31-24(19)37-17-6-7-18(37)13-36(2)12-17/h8-11,14-15,17-18H,3-7,12-13H2,1-2H3,(H,35,39)(H2,29,30,33,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470403
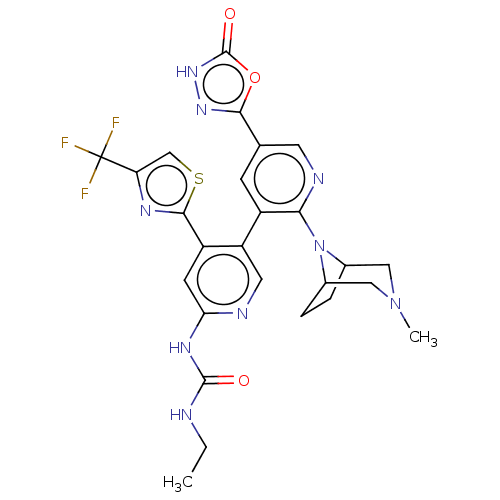
(CHEMBL4294467)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc(cnc1N1C2CCC1CN(C)C2)-c1n[nH]c(=O)o1 Show InChI InChI=1S/C26H26F3N9O3S/c1-3-30-24(39)34-20-7-17(23-33-19(12-42-23)26(27,28)29)18(9-31-20)16-6-13(22-35-36-25(40)41-22)8-32-21(16)38-14-4-5-15(38)11-37(2)10-14/h6-9,12,14-15H,3-5,10-11H2,1-2H3,(H,36,40)(H2,30,31,34,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470400
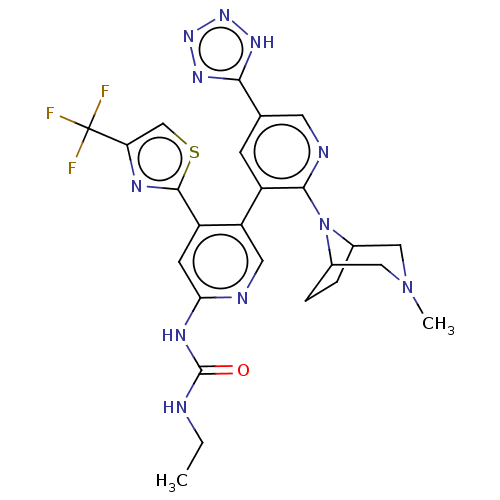
(CHEMBL4289998)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc(cnc1N1C2CCC1CN(C)C2)-c1nnn[nH]1 Show InChI InChI=1S/C25H26F3N11OS/c1-3-29-24(40)33-20-7-17(23-32-19(12-41-23)25(26,27)28)18(9-30-20)16-6-13(21-34-36-37-35-21)8-31-22(16)39-14-4-5-15(39)11-38(2)10-14/h6-9,12,14-15H,3-5,10-11H2,1-2H3,(H2,29,30,33,40)(H,34,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470394
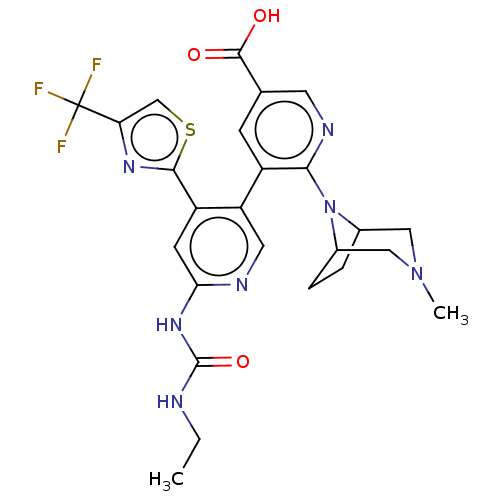
(CHEMBL4281775)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc(cnc1N1C2CCC1CN(C)C2)C(O)=O Show InChI InChI=1S/C25H26F3N7O3S/c1-3-29-24(38)33-20-7-17(22-32-19(12-39-22)25(26,27)28)18(9-30-20)16-6-13(23(36)37)8-31-21(16)35-14-4-5-15(35)11-34(2)10-14/h6-9,12,14-15H,3-5,10-11H2,1-2H3,(H,36,37)(H2,29,30,33,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470395
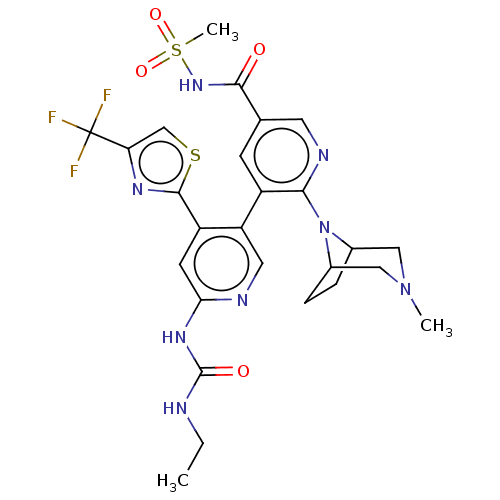
(CHEMBL4279313)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc(cnc1N1C2CCC1CN(C)C2)C(=O)NS(C)(=O)=O Show InChI InChI=1S/C26H29F3N8O4S2/c1-4-30-25(39)34-21-8-18(24-33-20(13-42-24)26(27,28)29)19(10-31-21)17-7-14(23(38)35-43(3,40)41)9-32-22(17)37-15-5-6-16(37)12-36(2)11-15/h7-10,13,15-16H,4-6,11-12H2,1-3H3,(H,35,38)(H2,30,31,34,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470405
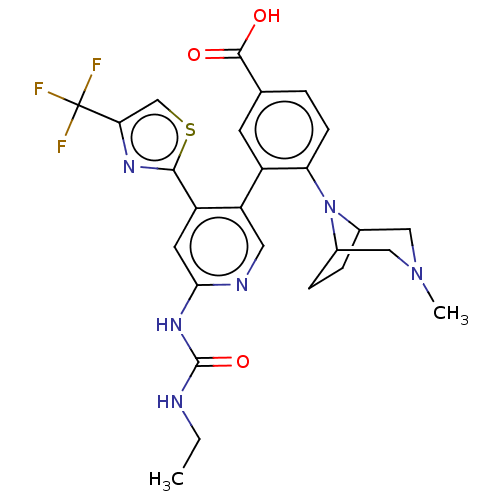
(CHEMBL4289650)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc(ccc1N1C2CCC1CN(C)C2)C(O)=O Show InChI InChI=1S/C26H27F3N6O3S/c1-3-30-25(38)33-22-9-18(23-32-21(13-39-23)26(27,28)29)19(10-31-22)17-8-14(24(36)37)4-7-20(17)35-15-5-6-16(35)12-34(2)11-15/h4,7-10,13,15-16H,3,5-6,11-12H2,1-2H3,(H,36,37)(H2,30,31,33,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470393
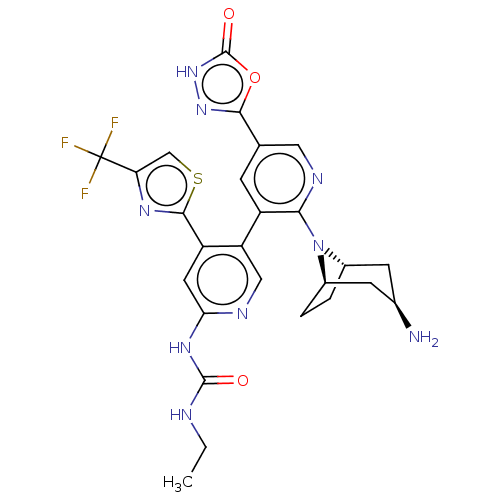
(CHEMBL4291061)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](N)C1)N2c1ncc(cc1-c1cnc(NC(=O)NCC)cc1-c1nc(cs1)C(F)(F)F)-c1n[nH]c(=O)o1 |r| Show InChI InChI=1S/C26H26F3N9O3S/c1-2-31-24(39)35-20-8-17(23-34-19(11-42-23)26(27,28)29)18(10-32-20)16-5-12(22-36-37-25(40)41-22)9-33-21(16)38-14-3-4-15(38)7-13(30)6-14/h5,8-11,13-15H,2-4,6-7,30H2,1H3,(H,37,40)(H2,31,32,35,39)/t13-,14+,15- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470404
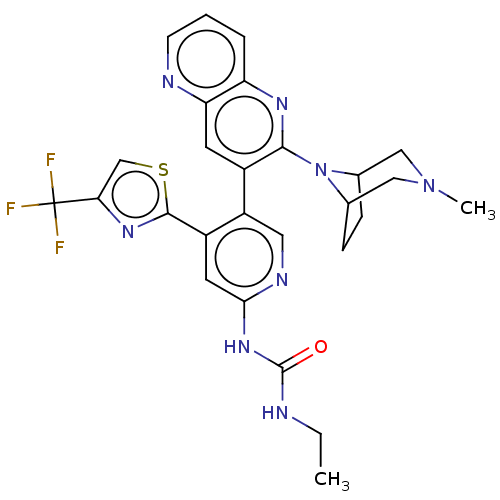
(CHEMBL4279382)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc2ncccc2nc1N1C2CCC1CN(C)C2 Show InChI InChI=1S/C27H27F3N8OS/c1-3-31-26(39)36-23-10-18(25-35-22(14-40-25)27(28,29)30)19(11-33-23)17-9-21-20(5-4-8-32-21)34-24(17)38-15-6-7-16(38)13-37(2)12-15/h4-5,8-11,14-16H,3,6-7,12-13H2,1-2H3,(H2,31,33,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470402
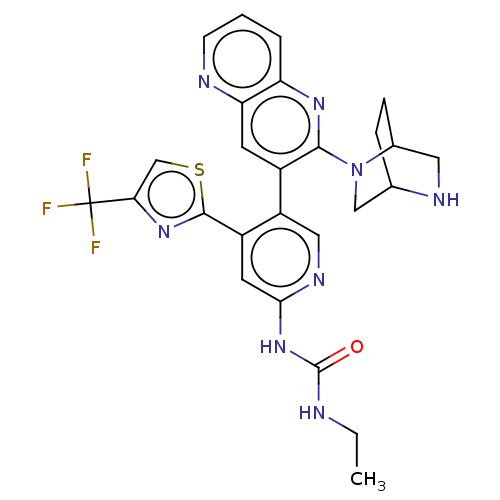
(CHEMBL4278317)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc2ncccc2nc1N1CC2CCC1CN2 Show InChI InChI=1S/C26H25F3N8OS/c1-2-30-25(38)36-22-9-17(24-35-21(13-39-24)26(27,28)29)18(11-33-22)16-8-20-19(4-3-7-31-20)34-23(16)37-12-14-5-6-15(37)10-32-14/h3-4,7-9,11,13-15,32H,2,5-6,10,12H2,1H3,(H2,30,33,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470399
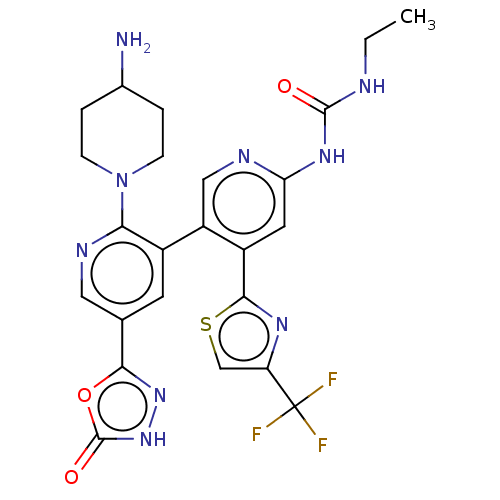
(CHEMBL4286573)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc(cnc1N1CCC(N)CC1)-c1n[nH]c(=O)o1 Show InChI InChI=1S/C24H24F3N9O3S/c1-2-29-22(37)33-18-8-15(21-32-17(11-40-21)24(25,26)27)16(10-30-18)14-7-12(20-34-35-23(38)39-20)9-31-19(14)36-5-3-13(28)4-6-36/h7-11,13H,2-6,28H2,1H3,(H,35,38)(H2,29,30,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470406
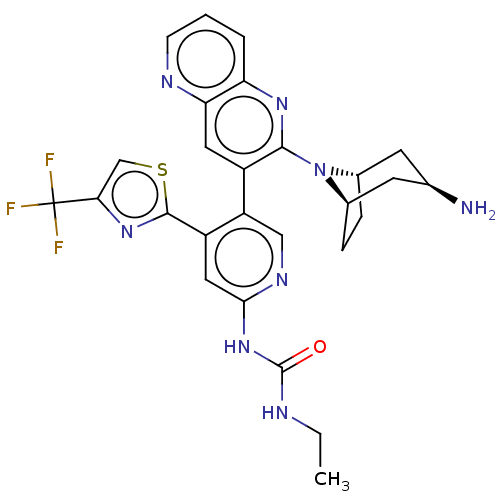
(CHEMBL4295081)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](N)C1)N2c1nc2cccnc2cc1-c1cnc(NC(=O)NCC)cc1-c1nc(cs1)C(F)(F)F |r| Show InChI InChI=1S/C27H27F3N8OS/c1-2-32-26(39)37-23-11-18(25-36-22(13-40-25)27(28,29)30)19(12-34-23)17-10-21-20(4-3-7-33-21)35-24(17)38-15-5-6-16(38)9-14(31)8-15/h3-4,7,10-16H,2,5-6,8-9,31H2,1H3,(H2,32,34,37,39)/t14-,15+,16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470398

(CHEMBL4286232)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc2ncccc2nc1N1CC2CCCC(C1)N2 |THB:30:31:39:34.35.36| Show InChI InChI=1S/C27H27F3N8OS/c1-2-31-26(39)37-23-10-18(25-36-22(14-40-25)27(28,29)30)19(11-33-23)17-9-21-20(7-4-8-32-21)35-24(17)38-12-15-5-3-6-16(13-38)34-15/h4,7-11,14-16,34H,2-3,5-6,12-13H2,1H3,(H2,31,33,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470396
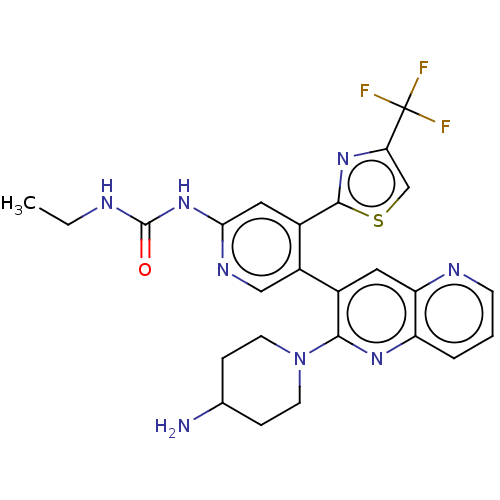
(CHEMBL4293447)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc2ncccc2nc1N1CCC(N)CC1 Show InChI InChI=1S/C25H25F3N8OS/c1-2-30-24(37)35-21-11-16(23-34-20(13-38-23)25(26,27)28)17(12-32-21)15-10-19-18(4-3-7-31-19)33-22(15)36-8-5-14(29)6-9-36/h3-4,7,10-14H,2,5-6,8-9,29H2,1H3,(H2,30,32,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50058509
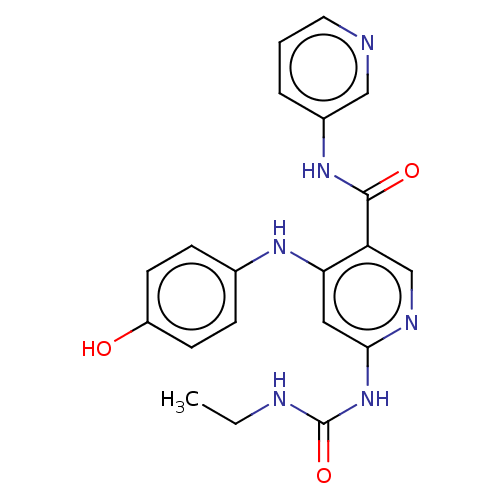
(CHEMBL3329317)Show SMILES CCNC(=O)Nc1cc(Nc2ccc(O)cc2)c(cn1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C20H20N6O3/c1-2-22-20(29)26-18-10-17(24-13-5-7-15(27)8-6-13)16(12-23-18)19(28)25-14-4-3-9-21-11-14/h3-12,27H,2H2,1H3,(H,25,28)(H3,22,23,24,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus ParE |
Eur J Med Chem 86: 31-8 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.025
BindingDB Entry DOI: 10.7270/Q2S75J0P |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50393080

(CHEMBL2152856)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)OP([O-])([O-])=O |r| Show InChI InChI=1S/C21H26FN6O6P/c1-4-23-20(29)28-19-26-13-8-12(16(22)15(17(13)27-19)14-6-5-7-33-14)11-9-24-18(25-10-11)21(2,3)34-35(30,31)32/h8-10,14H,4-7H2,1-3H3,(H2,30,31,32)(H3,23,26,27,28,29)/p-2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA topoisomerase 4 Par E subunit by ATPase assay |
ACS Med Chem Lett 3: 783-784 (2012)
Article DOI: 10.1021/ml300234y
BindingDB Entry DOI: 10.7270/Q2N29Z1N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50173879
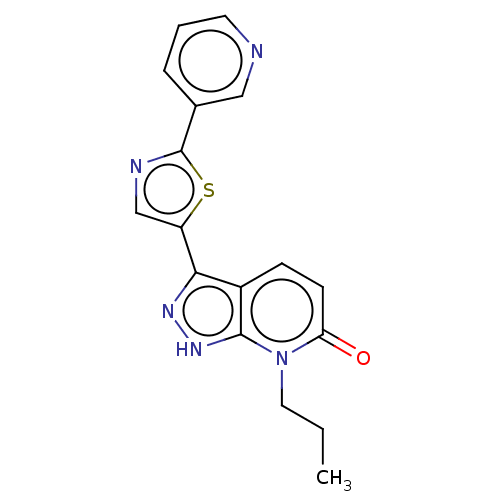
(CHEMBL3810359)Show InChI InChI=1S/C17H15N5OS/c1-2-8-22-14(23)6-5-12-15(20-21-16(12)22)13-10-19-17(24-13)11-4-3-7-18-9-11/h3-7,9-10H,2,8H2,1H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA parE ATPase activity incubated for 30 mins by fluorescence polarization assay |
ACS Med Chem Lett 7: 374-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00368
BindingDB Entry DOI: 10.7270/Q20R9RB6 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470401

(CHEMBL4290062)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc2ncccc2nc1N1C2CC1CN(C)C2 Show InChI InChI=1S/C26H25F3N8OS/c1-3-30-25(38)35-22-9-17(24-34-21(13-39-24)26(27,28)29)18(10-32-22)16-8-20-19(5-4-6-31-20)33-23(16)37-14-7-15(37)12-36(2)11-14/h4-6,8-10,13-15H,3,7,11-12H2,1-2H3,(H2,30,32,35,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470397
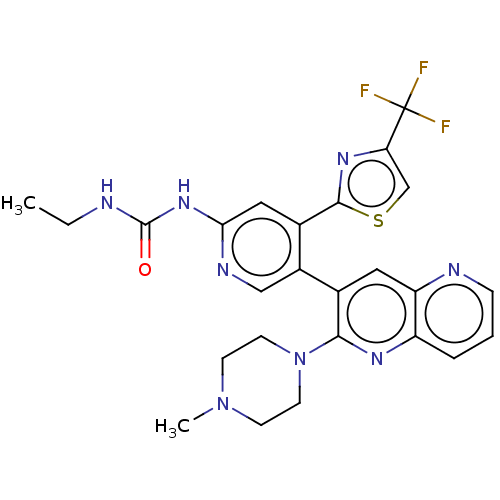
(CHEMBL4294536)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cc2ncccc2nc1N1CCN(C)CC1 Show InChI InChI=1S/C25H25F3N8OS/c1-3-29-24(37)34-21-12-16(23-33-20(14-38-23)25(26,27)28)17(13-31-21)15-11-19-18(5-4-6-30-19)32-22(15)36-9-7-35(2)8-10-36/h4-6,11-14H,3,7-10H2,1-2H3,(H2,29,31,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50006565
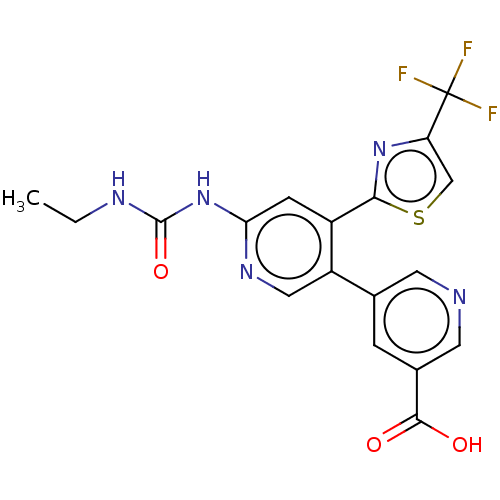
(CHEMBL3235085)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cncc(c1)C(O)=O Show InChI InChI=1S/C18H14F3N5O3S/c1-2-23-17(29)26-14-4-11(15-25-13(8-30-15)18(19,20)21)12(7-24-14)9-3-10(16(27)28)6-22-5-9/h3-8H,2H2,1H3,(H,27,28)(H2,23,24,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50173878

(CHEMBL3809316)Show InChI InChI=1S/C16H13N5OS/c1-2-21-13(22)6-5-11-14(19-20-15(11)21)12-9-18-16(23-12)10-4-3-7-17-8-10/h3-9H,2H2,1H3,(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 746 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA parE ATPase activity incubated for 30 mins by fluorescence polarization assay |
ACS Med Chem Lett 7: 374-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00368
BindingDB Entry DOI: 10.7270/Q20R9RB6 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50058511

(CHEMBL3329318)Show SMILES CCNC(=O)Nc1cc(Nc2cccc(O)c2)c(cn1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C20H20N6O3/c1-2-22-20(29)26-18-10-17(24-13-5-3-7-15(27)9-13)16(12-23-18)19(28)25-14-6-4-8-21-11-14/h3-12,27H,2H2,1H3,(H,25,28)(H3,22,23,24,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 86: 31-8 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.025
BindingDB Entry DOI: 10.7270/Q2S75J0P |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50058510
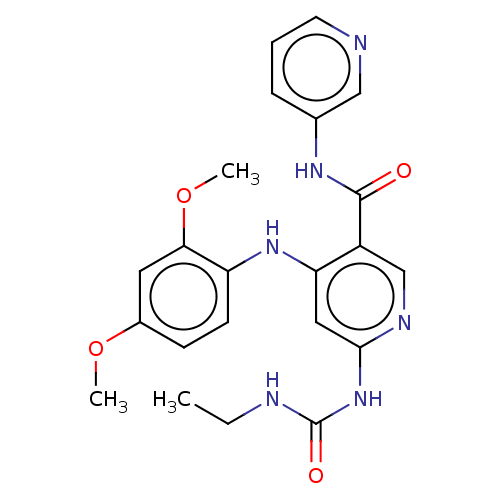
(CHEMBL3329319)Show SMILES CCNC(=O)Nc1cc(Nc2ccc(OC)cc2OC)c(cn1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C22H24N6O4/c1-4-24-22(30)28-20-11-18(27-17-8-7-15(31-2)10-19(17)32-3)16(13-25-20)21(29)26-14-6-5-9-23-12-14/h5-13H,4H2,1-3H3,(H,26,29)(H3,24,25,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus ParE |
Eur J Med Chem 86: 31-8 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.025
BindingDB Entry DOI: 10.7270/Q2S75J0P |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50058509

(CHEMBL3329317)Show SMILES CCNC(=O)Nc1cc(Nc2ccc(O)cc2)c(cn1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C20H20N6O3/c1-2-22-20(29)26-18-10-17(24-13-5-7-15(27)8-6-13)16(12-23-18)19(28)25-14-4-3-9-21-11-14/h3-12,27H,2H2,1H3,(H,25,28)(H3,22,23,24,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 86: 31-8 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.025
BindingDB Entry DOI: 10.7270/Q2S75J0P |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50470407

(CHEMBL4286625)Show SMILES CCNC(=O)Nc1cc(-c2nc(cs2)C(F)(F)F)c(cn1)-c1cnc2cccnc2c1 Show InChI InChI=1S/C20H15F3N6OS/c1-2-24-19(30)29-17-7-12(18-28-16(10-31-18)20(21,22)23)13(9-27-17)11-6-15-14(26-8-11)4-3-5-25-15/h3-10H,2H2,1H3,(H2,24,27,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 157: 610-621 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.025
BindingDB Entry DOI: 10.7270/Q28P637N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50226181
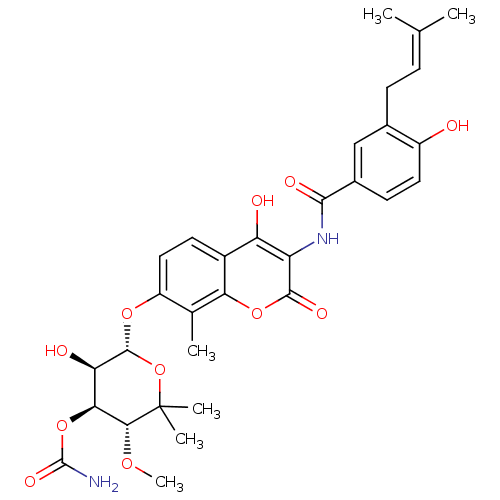
((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@@H](-[#8])-[#6@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] |r| Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25+,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Staphylococcus aures ParE using pBR322 DNA as substrate in presence of ATP |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.112022
BindingDB Entry DOI: 10.7270/Q2CC149W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 4 subunit B
(Escherichia coli (strain K12)) | BDBM50058510

(CHEMBL3329319)Show SMILES CCNC(=O)Nc1cc(Nc2ccc(OC)cc2OC)c(cn1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C22H24N6O4/c1-4-24-22(30)28-20-11-18(27-17-8-7-15(31-2)10-19(17)32-3)16(13-25-20)21(29)26-14-6-5-9-23-12-14/h5-13H,4H2,1-3H3,(H,26,29)(H3,24,25,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ParE |
Eur J Med Chem 86: 31-8 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.025
BindingDB Entry DOI: 10.7270/Q2S75J0P |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit B
(Staphylococcus aureus) | BDBM50550961

(CHEMBL4764715)Show SMILES CCn1c2ccccc2c(O)c(C(=O)Nc2nnc(s2)-c2nccs2)c1=O | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Staphylococcus aures ParE using pBR322 DNA as substrate in presence of ATP |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.112022
BindingDB Entry DOI: 10.7270/Q2CC149W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data