

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304170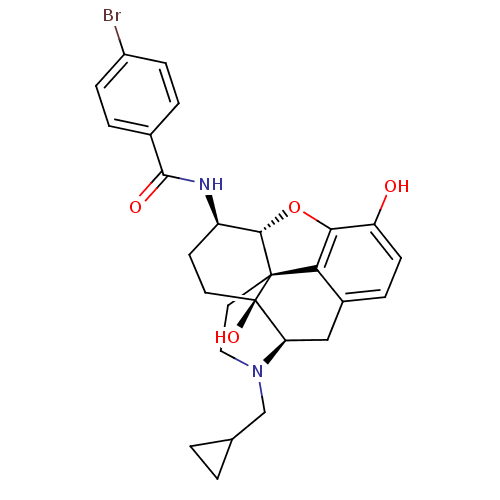 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards Wild-type kappa opioid receptor expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002186 (CHEMBL440987 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Lys...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50378578 (CHEMBL1627119) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50102711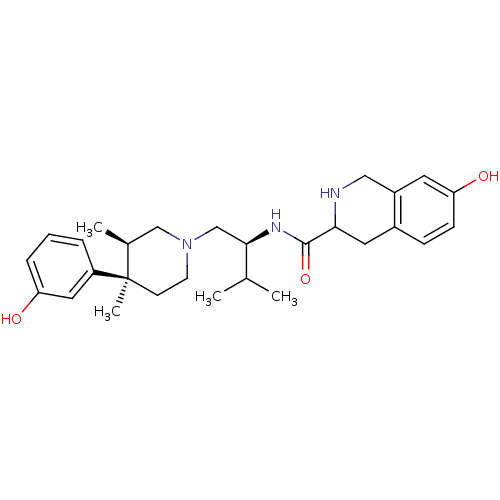 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (U50,488) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor kappa 1 | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002182 (CHEMBL415617 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Lys...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50242686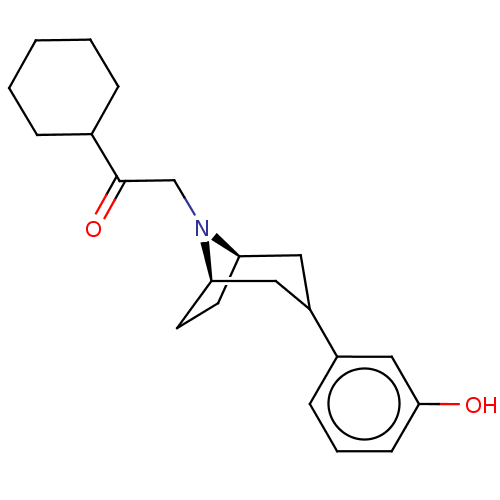 (CHEMBL4077003) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DPN from guinea pig kappa opioid receptor expressed in CHOK1 cell membranes | Bioorg Med Chem Lett 27: 2926-2930 (2017) Article DOI: 10.1016/j.bmcl.2017.04.092 BindingDB Entry DOI: 10.7270/Q2WD430K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714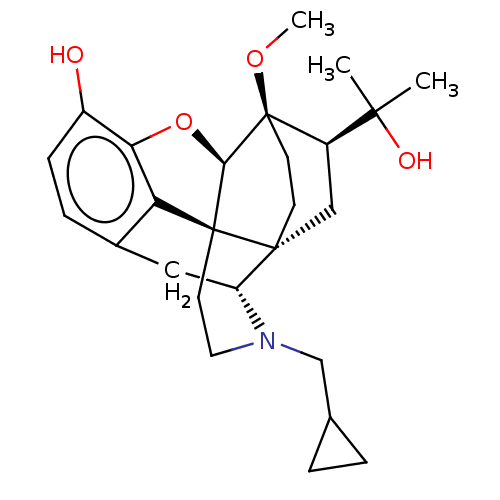 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by PDSP Ki Database | Mol Pharmacol 45: 330-4 (1994) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q21Z42X1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50229232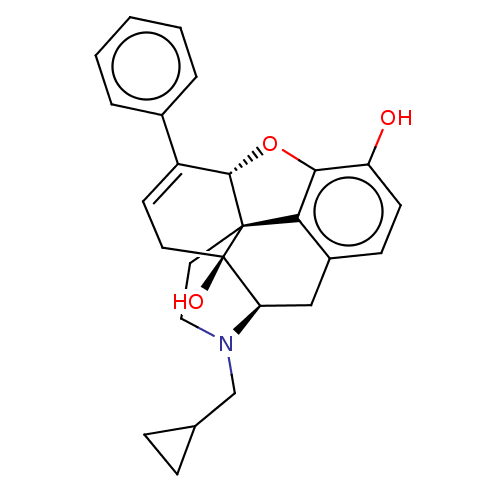 (CHEMBL610527) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to opioid receptor kappa using [3H]-EK as radioligand in guinea pig brain membrane | J Med Chem 34: 1715-20 (1991) BindingDB Entry DOI: 10.7270/Q2T43TPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50242686 (CHEMBL4077003) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma US, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DPN from guinea pig kappa opioid receptor expressed in CHOK1 cell membranes | Bioorg Med Chem Lett 27: 2926-2930 (2017) Article DOI: 10.1016/j.bmcl.2017.04.092 BindingDB Entry DOI: 10.7270/Q2WD430K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199410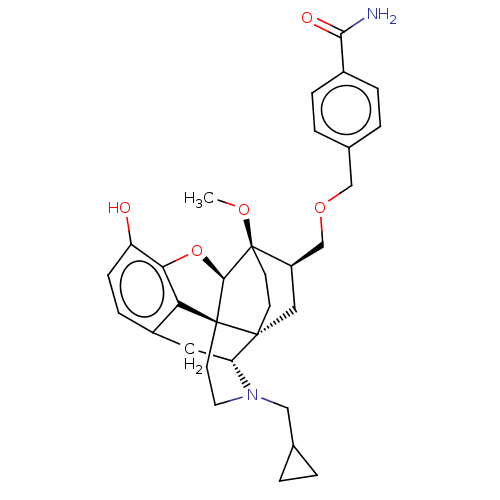 (US9221831, 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430590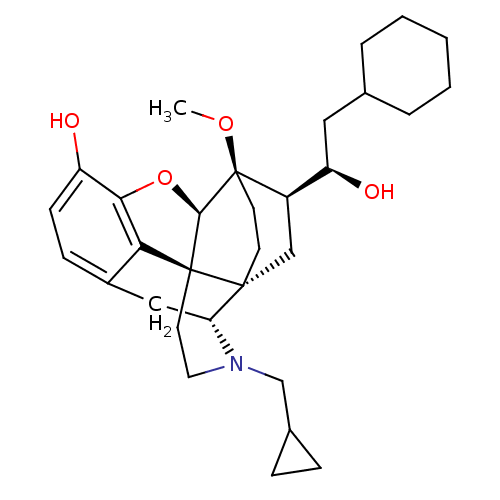 (CHEMBL2338742) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304175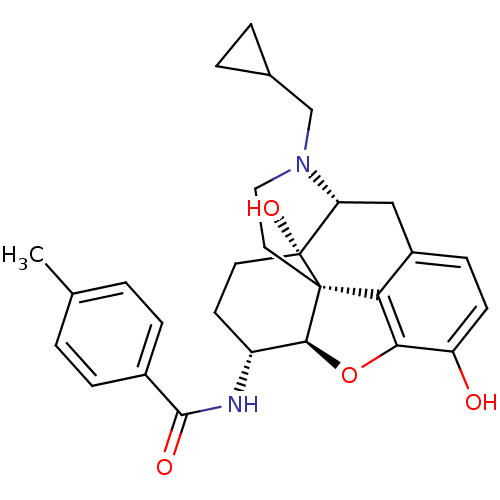 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50577958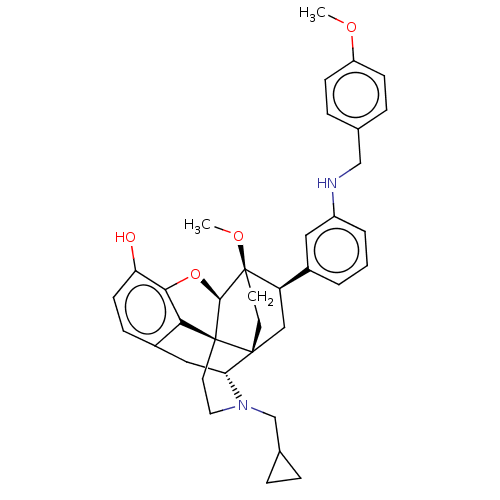 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240938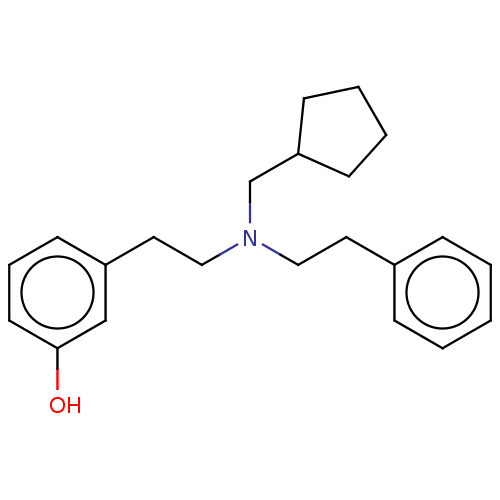 (CHEMBL4071862) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325534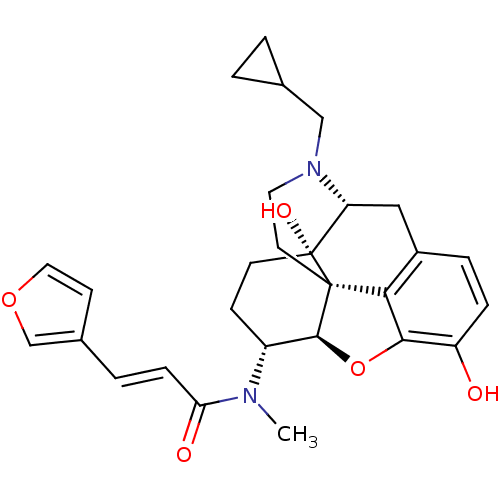 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430589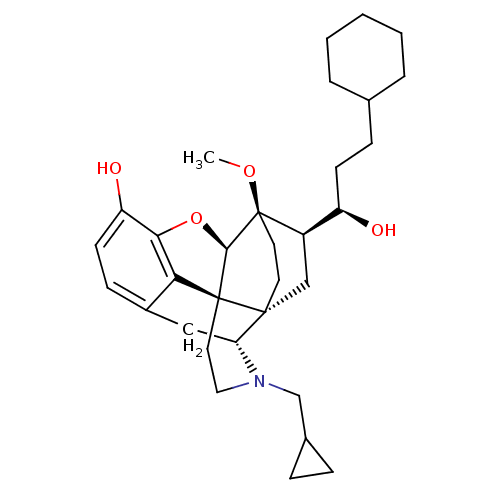 (CHEMBL2338716) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by PDSP Ki Database | Mol Pharmacol 45: 330-4 (1994) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q21Z42X1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199350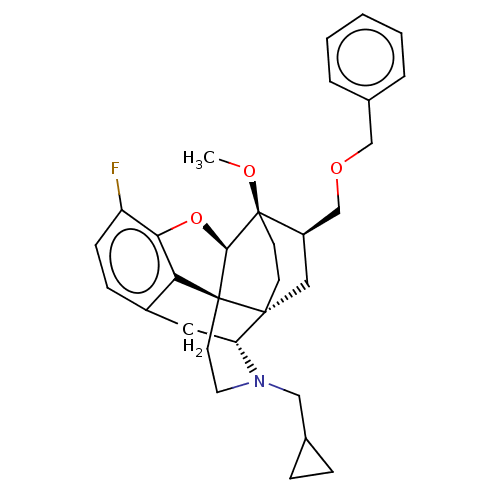 (US9221831, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430588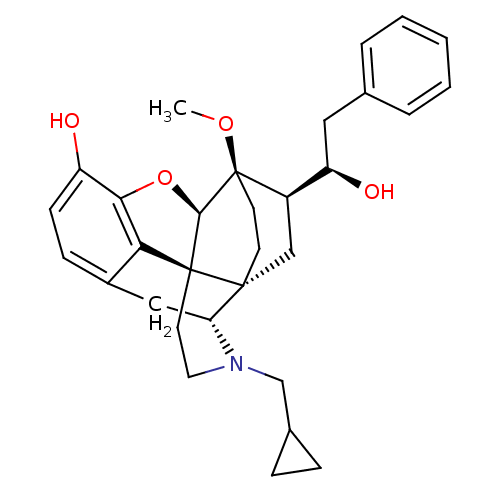 (CHEMBL2338717) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002181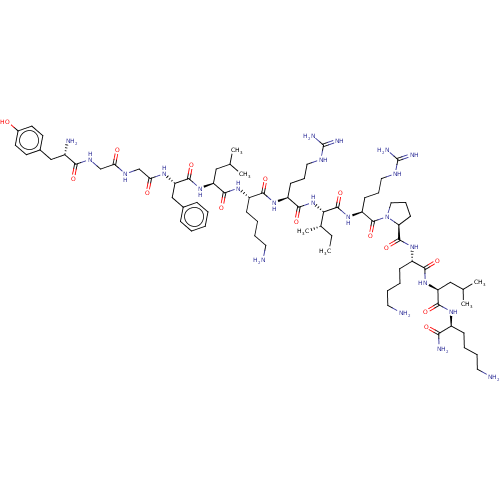 (CHEMBL266717 | Tyr-Gly-Gly-Phe-Leu-Lys-Arg-Ile-Arg...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059585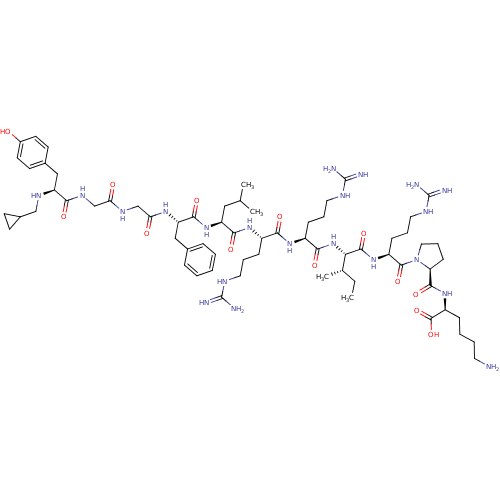 (CHEMBL442323 | N-CPM[D-Pro-10]Dyn A-(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002347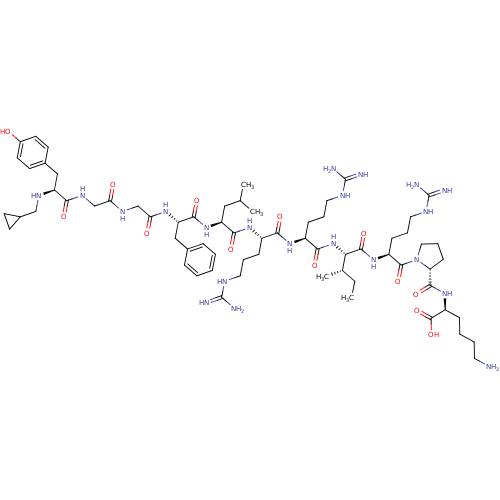 (CHEMBL425806 | [N-cyclopropyl methylTyr1, D-pro10]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001714 (2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo Curated by ChEMBL | Assay Description Binding affinity to human cloned kappa opioid receptor expressed in HEK293 cells by radioligand displacement assay | J Med Chem 57: 5464-9 (2014) Article DOI: 10.1021/jm500503k BindingDB Entry DOI: 10.7270/Q2RB764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor in guinea pig caudate assessed as inhibition of U69593-stimulated [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002183 (CHEMBL266515 | Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Ile-Arg...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551314 (CHEMBL4751305) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002184 (CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002185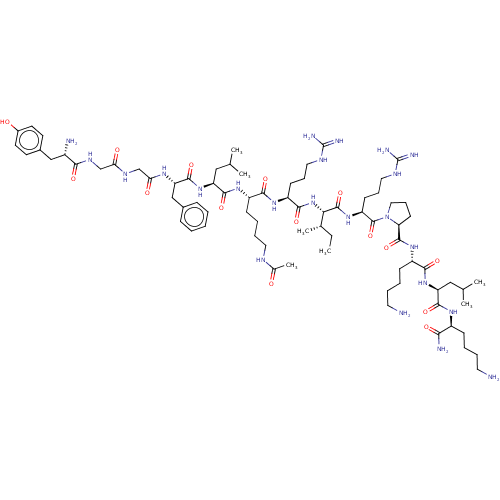 (CHEMBL438741 | Tyr-Gly-Gly-Phe-Leu-Lys(Ac)-Arg-Ile...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50551311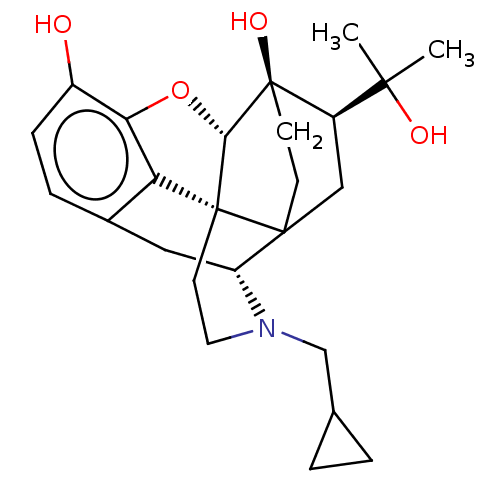 (CHEMBL4746512) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112145 BindingDB Entry DOI: 10.7270/Q2W66QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50430615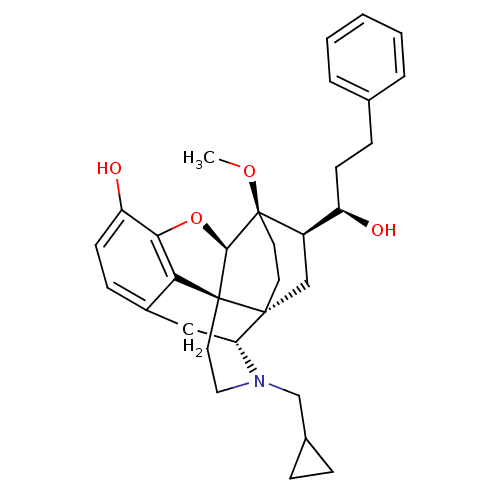 (CHEMBL2338718) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human KOR expressed in CHO cells | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM86549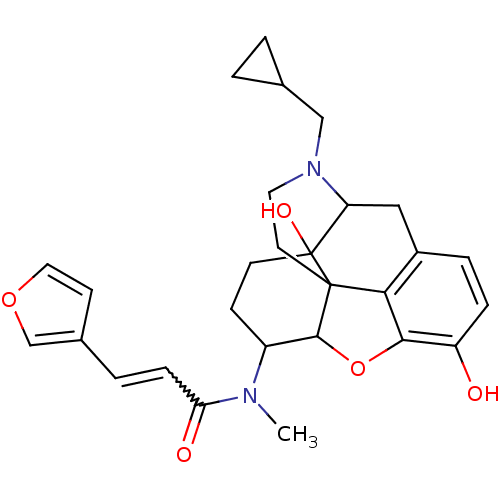 (CAS_213055 | NSC_213055 | TRK-820) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 220-30 (2005) Article DOI: 10.1124/jpet.104.073668 BindingDB Entry DOI: 10.7270/Q26T0K6G | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364547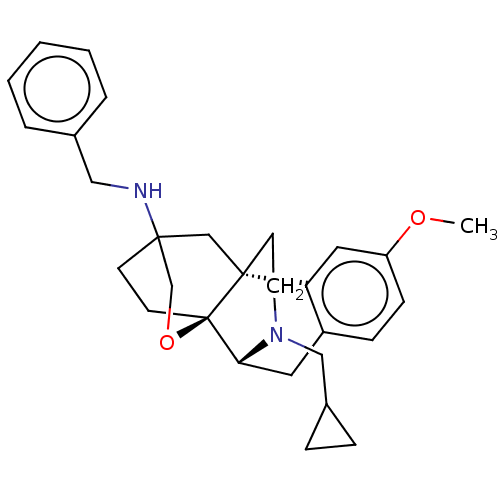 ((4bR,6S,8aS,9R)-N-benzyl-11- (cyclopropylmethyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Activity at human cloned kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50596292 (CHEMBL5185211) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059583 (CHEMBL268818 | N-benzyl[D-Pro-10]Dyn A-(1-11) | Ph...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002345 (CHEMBL407529 | PhCH2Tyr-Gly-Gly-Phe-Leu-Arg-Arg-ll...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM83436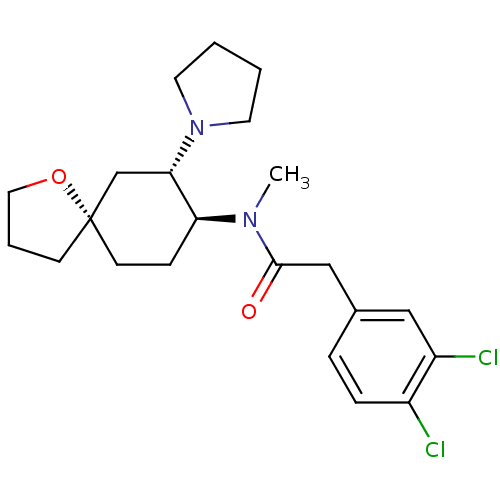 (2-(3,4-dichlorophenyl)-N-methyl-N-[(5R,7S,8S)-7-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by PDSP Ki Database | Mol Pharmacol 45: 330-4 (1994) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q21Z42X1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50303629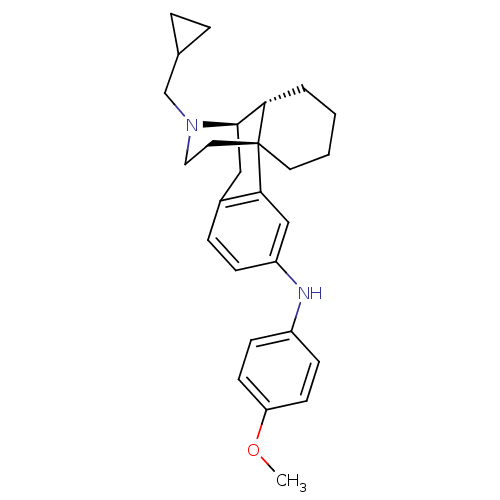 (17-(Cyclopropylmethyl)-N-(4-methoxyphenyl)morphina...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 53: 402-18 (2010) Article DOI: 10.1021/jm9013482 BindingDB Entry DOI: 10.7270/Q2668D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002346 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human recombinant mu opioid receptor expressed in HEK cells after 60 mins | Eur J Med Chem 90: 742-50 (2015) Article DOI: 10.1016/j.ejmech.2014.12.016 BindingDB Entry DOI: 10.7270/Q2TQ636H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002346 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50346951 (CHEMBL1795711 | CHEMBL1795714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-BNtxA from mouse cloned KOR-1 expressed in CHO cell membrane after 90 mins | J Med Chem 55: 6352-62 (2012) Article DOI: 10.1021/jm300305c BindingDB Entry DOI: 10.7270/Q2930V8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50013388 (6-Ethyl-3-(1-hydroxy-cyclopropylmethyl)-11,11-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by PDSP Ki Database | NIDA Res Monogr 178: 440-66 (1998) BindingDB Entry DOI: 10.7270/Q23J3BH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50347177 (CHEMBL1797687) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum homogenate by scintillation counting | Bioorg Med Chem Lett 21: 4104-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.147 BindingDB Entry DOI: 10.7270/Q25M66P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 14670 total ) | Next | Last >> |