 Found 20502 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 1'
Found 20502 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50114862
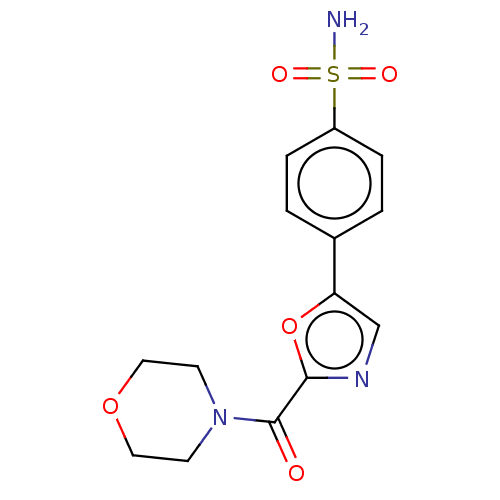
(CHEMBL3608874)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cnc(o1)C(=O)N1CCOCC1 Show InChI InChI=1S/C14H15N3O5S/c15-23(19,20)11-3-1-10(2-4-11)12-9-16-13(22-12)14(18)17-5-7-21-8-6-17/h1-4,9H,5-8H2,(H2,15,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method |
Eur J Med Chem 101: 334-47 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.022
BindingDB Entry DOI: 10.7270/Q2W95BZC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50114861
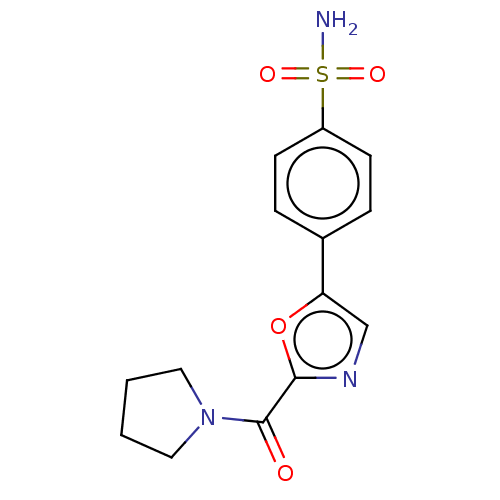
(CHEMBL3608873)Show InChI InChI=1S/C14H15N3O4S/c15-22(19,20)11-5-3-10(4-6-11)12-9-16-13(21-12)14(18)17-7-1-2-8-17/h3-6,9H,1-2,7-8H2,(H2,15,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method |
Eur J Med Chem 101: 334-47 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.022
BindingDB Entry DOI: 10.7270/Q2W95BZC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26994
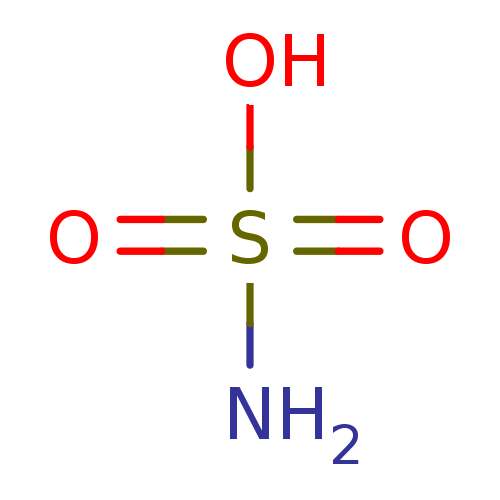
(CHEMBL68253 | H2NSO3H | sulfamic acid)Show InChI InChI=1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA1 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093585
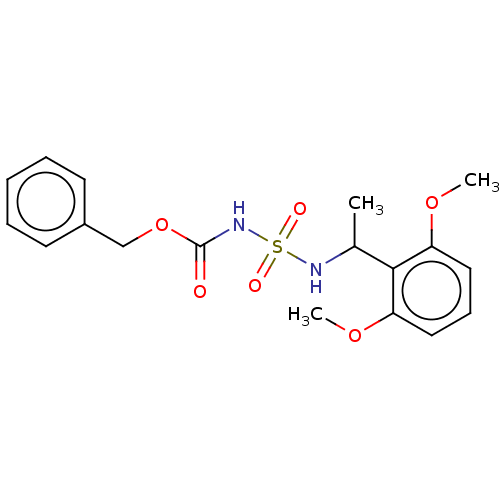
(CHEMBL3585779)Show SMILES COc1cccc(OC)c1C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(17-15(24-2)10-7-11-16(17)25-3)19-27(22,23)20-18(21)26-12-14-8-5-4-6-9-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093587
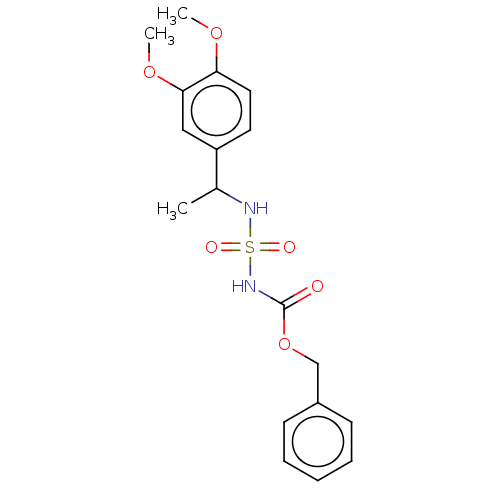
(CHEMBL3585777)Show SMILES COc1ccc(cc1OC)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(15-9-10-16(24-2)17(11-15)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50114853
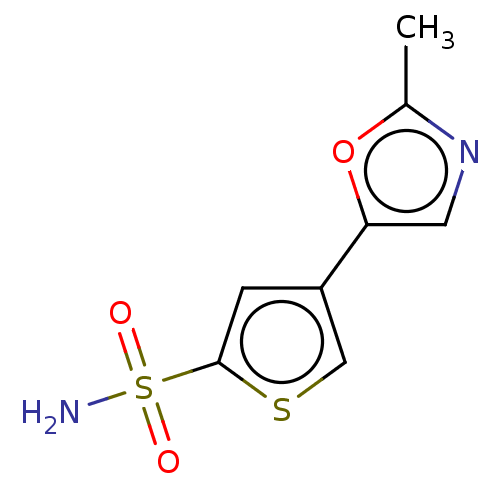
(CHEMBL3608890)Show InChI InChI=1S/C8H8N2O3S2/c1-5-10-3-7(13-5)6-2-8(14-4-6)15(9,11)12/h2-4H,1H3,(H2,9,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method |
Eur J Med Chem 101: 334-47 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.022
BindingDB Entry DOI: 10.7270/Q2W95BZC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50114857
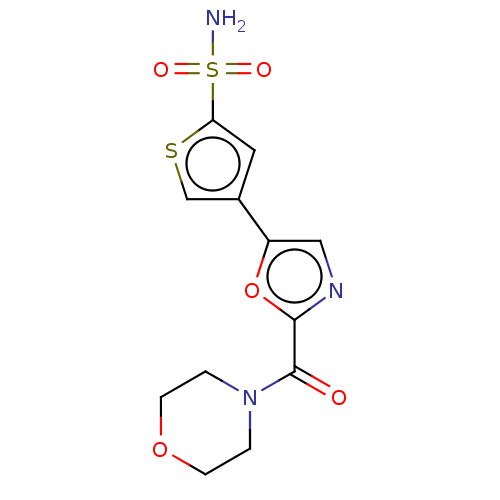
(CHEMBL3608894)Show InChI InChI=1S/C12H13N3O5S2/c13-22(17,18)10-5-8(7-21-10)9-6-14-11(20-9)12(16)15-1-3-19-4-2-15/h5-7H,1-4H2,(H2,13,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method |
Eur J Med Chem 101: 334-47 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.022
BindingDB Entry DOI: 10.7270/Q2W95BZC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50329836
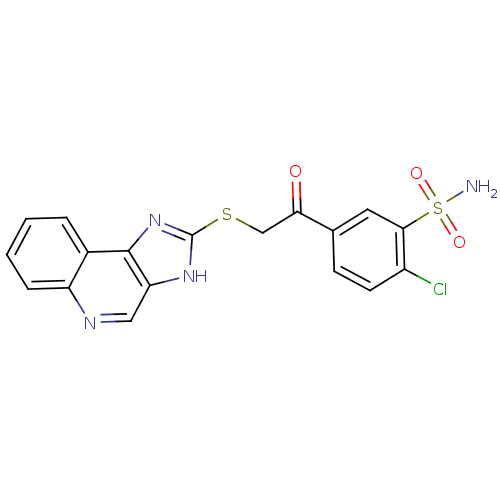
(2-Chloro-5-[(1H-imidazo[4,5-c]quinolin-2-ylsulfany...)Show SMILES NS(=O)(=O)c1cc(ccc1Cl)C(=O)CSc1nc2c(cnc3ccccc23)[nH]1 Show InChI InChI=1S/C18H13ClN4O3S2/c19-12-6-5-10(7-16(12)28(20,25)26)15(24)9-27-18-22-14-8-21-13-4-2-1-3-11(13)17(14)23-18/h1-8H,9H2,(H,22,23)(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114247
BindingDB Entry DOI: 10.7270/Q2MC941H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50252993
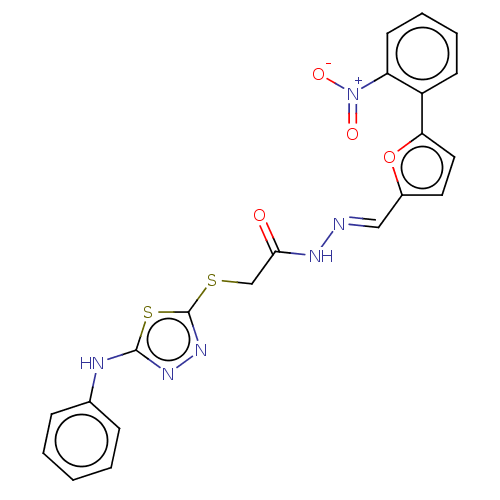
(CHEMBL4073678)Show SMILES [O-][N+](=O)c1ccccc1-c1ccc(\C=N\NC(=O)CSc2nnc(Nc3ccccc3)s2)o1 Show InChI InChI=1S/C21H16N6O4S2/c28-19(13-32-21-26-25-20(33-21)23-14-6-2-1-3-7-14)24-22-12-15-10-11-18(31-15)16-8-4-5-9-17(16)27(29)30/h1-12H,13H2,(H,23,25)(H,24,28)/b22-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr.
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... |
Bioorg Med Chem 25: 3547-3554 (2017)
Article DOI: 10.1016/j.bmc.2017.05.005
BindingDB Entry DOI: 10.7270/Q28G8P41 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50252992
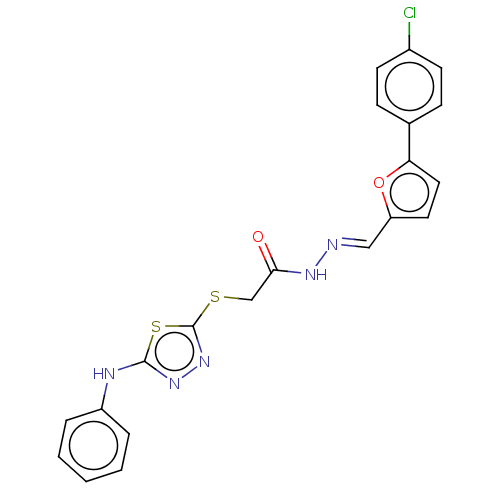
(CHEMBL4100496)Show SMILES Clc1ccc(cc1)-c1ccc(\C=N\NC(=O)CSc2nnc(Nc3ccccc3)s2)o1 Show InChI InChI=1S/C21H16ClN5O2S2/c22-15-8-6-14(7-9-15)18-11-10-17(29-18)12-23-25-19(28)13-30-21-27-26-20(31-21)24-16-4-2-1-3-5-16/h1-12H,13H2,(H,24,26)(H,25,28)/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr.
Curated by ChEMBL
| Assay Description
Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... |
Bioorg Med Chem 25: 3547-3554 (2017)
Article DOI: 10.1016/j.bmc.2017.05.005
BindingDB Entry DOI: 10.7270/Q28G8P41 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093589
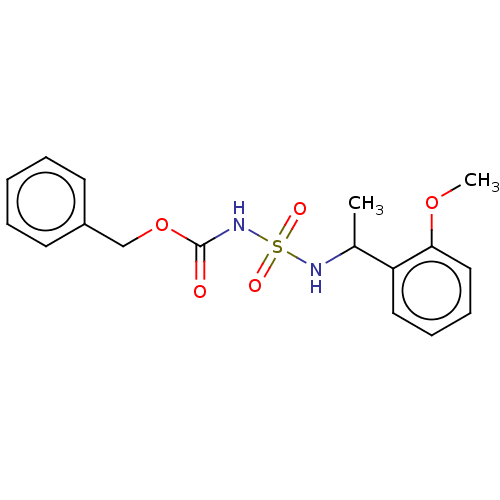
(CHEMBL3585775)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-10-6-7-11-16(15)23-2)18-25(21,22)19-17(20)24-12-14-8-4-3-5-9-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093588
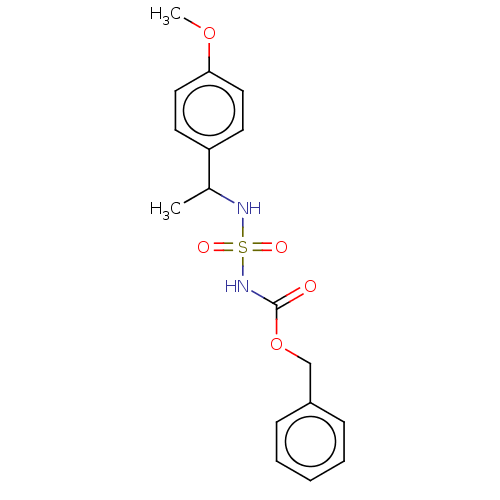
(CHEMBL3585776)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-8-10-16(23-2)11-9-15)18-25(21,22)19-17(20)24-12-14-6-4-3-5-7-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093586
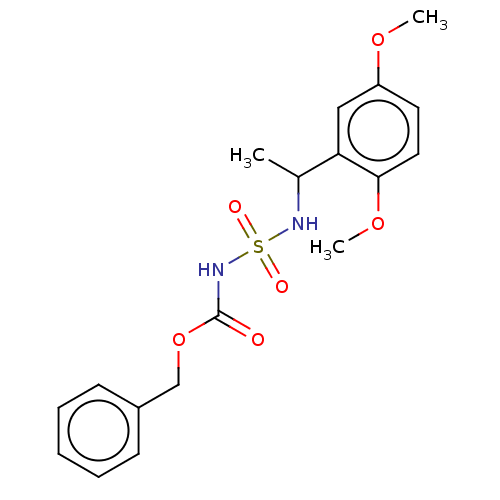
(CHEMBL3585778)Show SMILES COc1ccc(OC)c(c1)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(16-11-15(24-2)9-10-17(16)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50025094
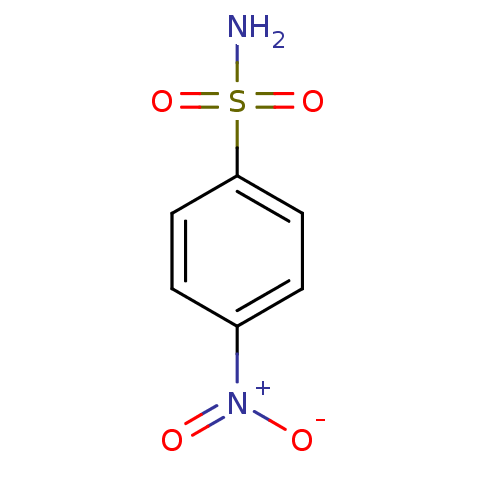
(4-Nitro-benzenesulfonamide | 4-nitrobenzenesulfona...)Show InChI InChI=1S/C6H6N2O4S/c7-13(11,12)6-3-1-5(2-4-6)8(9)10/h1-4H,(H2,7,11,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Carbonic anhydrase |
J Med Chem 29: 1488-94 (1986)
BindingDB Entry DOI: 10.7270/Q2HQ424N |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50492460
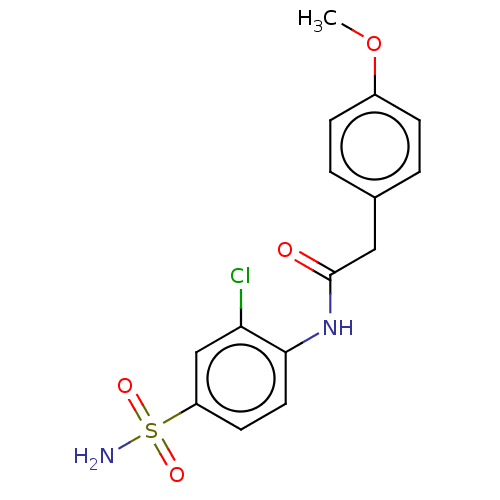
(CHEMBL2402776)Show InChI InChI=1S/C15H15ClN2O4S/c1-22-11-4-2-10(3-5-11)8-15(19)18-14-7-6-12(9-13(14)16)23(17,20)21/h2-7,9H,8H2,1H3,(H,18,19)(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 5228-32 (2013)
Article DOI: 10.1016/j.bmc.2013.06.029
BindingDB Entry DOI: 10.7270/Q2CJ8HFM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 preincubated for 15 mins by stopped flow CO2 dehydration method |
Bioorg Med Chem 24: 104-12 (2016)
Article DOI: 10.1016/j.bmc.2015.11.031
BindingDB Entry DOI: 10.7270/Q2222XRQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133408
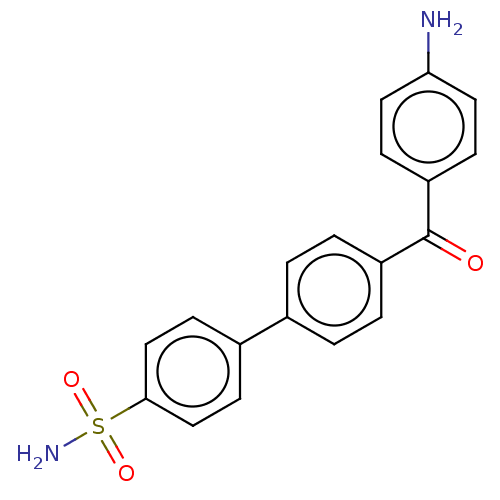
(CHEMBL3632844)Show SMILES Nc1ccc(cc1)C(=O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H16N2O3S/c20-17-9-5-16(6-10-17)19(22)15-3-1-13(2-4-15)14-7-11-18(12-8-14)25(21,23)24/h1-12H,20H2,(H2,21,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50555722
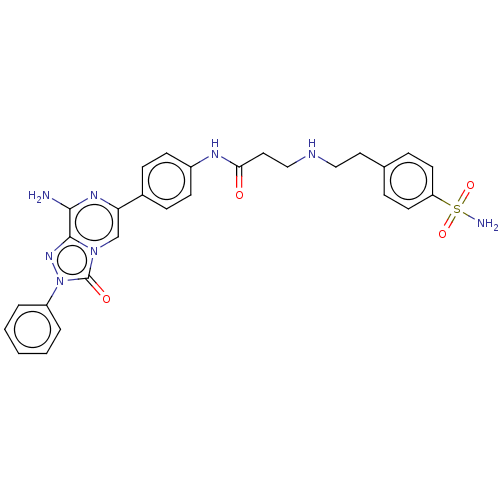
(CHEMBL4793920)Show SMILES Nc1nc(cn2c1nn(-c1ccccc1)c2=O)-c1ccc(NC(=O)CCNCCc2ccc(cc2)S(N)(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CA12 incubated for 15 mins by stopped- flow CO2 hydrase assay method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112478
BindingDB Entry DOI: 10.7270/Q2571GPQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093584
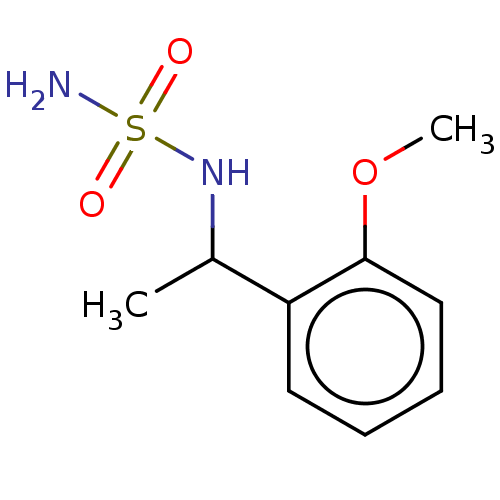
(CHEMBL3585780)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-5-3-4-6-9(8)14-2/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860
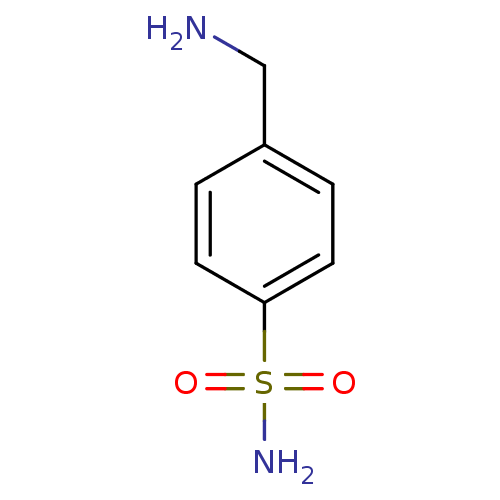
(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860

(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Ki value against human carbonic anhydrase XII (hCA XII) |
Bioorg Med Chem Lett 15: 963-9 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.053
BindingDB Entry DOI: 10.7270/Q29887RZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50025093
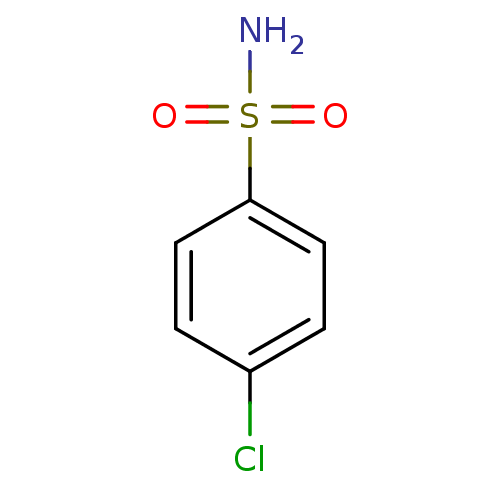
(4-Chloro-benzenesulfonamide | CHEMBL804 | P-Chloro...)Show InChI InChI=1S/C6H6ClNO2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,(H2,8,9,10) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Carbonic anhydrase |
J Med Chem 29: 1488-94 (1986)
BindingDB Entry DOI: 10.7270/Q2HQ424N |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860

(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by stopped flow CO2 hydration assay |
J Med Chem 53: 2913-26 (2010)
Article DOI: 10.1021/jm901888x
BindingDB Entry DOI: 10.7270/Q2DN460W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860

(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School
Curated by ChEMBL
| Assay Description
Inhibitory constant against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3828-33 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.055
BindingDB Entry DOI: 10.7270/Q2C82B2K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26995
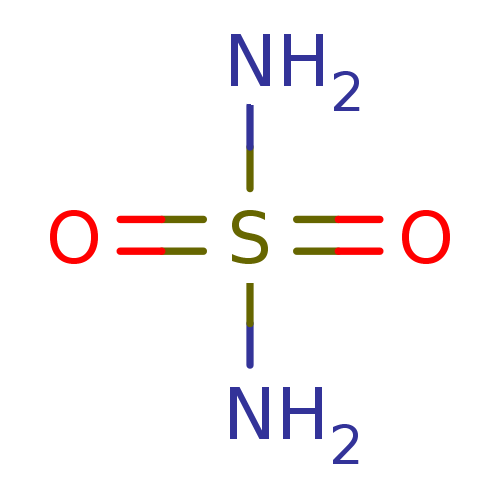
(CHEMBL355001 | H2NSO2NH2 | sulfamamide | sulfamide...)Show InChI InChI=1S/H4N2O2S/c1-5(2,3)4/h(H4,1,2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA1 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM11028
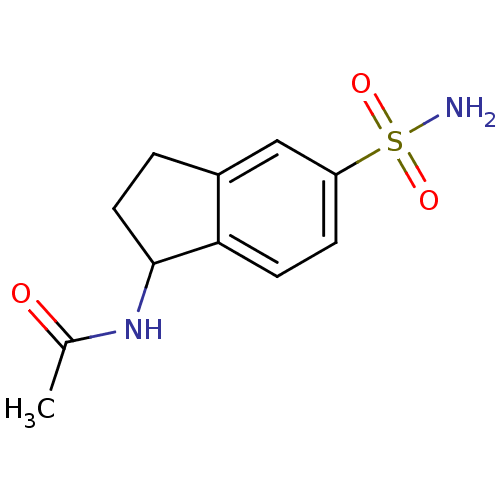
(1-Acetamido-5-sulfonamidoindane | CHEMBL359768 | I...)Show InChI InChI=1S/C11H14N2O3S/c1-7(14)13-11-5-2-8-6-9(17(12,15)16)3-4-10(8)11/h3-4,6,11H,2,5H2,1H3,(H,13,14)(H2,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 by CO2 hydration assay |
Eur J Med Chem 43: 2853-60 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.018
BindingDB Entry DOI: 10.7270/Q2Z03922 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133390
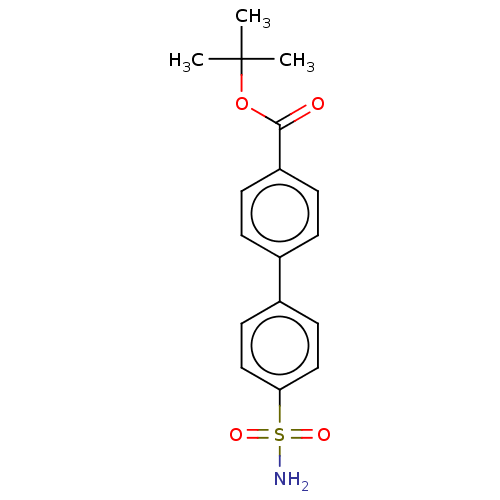
(CHEMBL3632826)Show SMILES CC(C)(C)OC(=O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H19NO4S/c1-17(2,3)22-16(19)14-6-4-12(5-7-14)13-8-10-15(11-9-13)23(18,20)21/h4-11H,1-3H3,(H2,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50153971
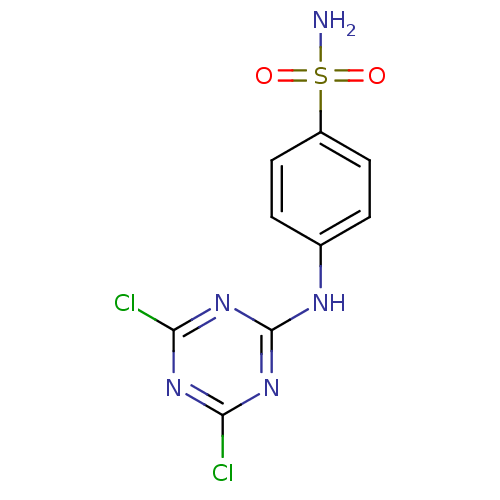
(4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C9H7Cl2N5O2S/c10-7-14-8(11)16-9(15-7)13-5-1-3-6(4-2-5)19(12,17)18/h1-4H,(H2,12,17,18)(H,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50155554
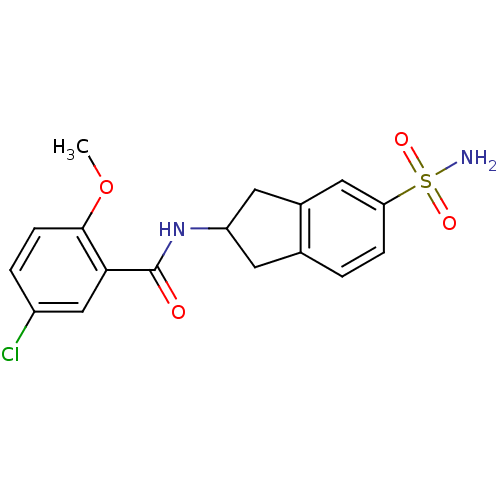
(5-Chloro-2-methoxy-N-(5-sulfamoyl-indan-2-yl)-benz...)Show SMILES COc1ccc(Cl)cc1C(=O)NC1Cc2ccc(cc2C1)S(N)(=O)=O Show InChI InChI=1S/C17H17ClN2O4S/c1-24-16-5-3-12(18)9-15(16)17(21)20-13-6-10-2-4-14(25(19,22)23)8-11(10)7-13/h2-5,8-9,13H,6-7H2,1H3,(H,20,21)(H2,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 by CO2 hydration assay |
Eur J Med Chem 43: 2853-60 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.018
BindingDB Entry DOI: 10.7270/Q2Z03922 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50566713
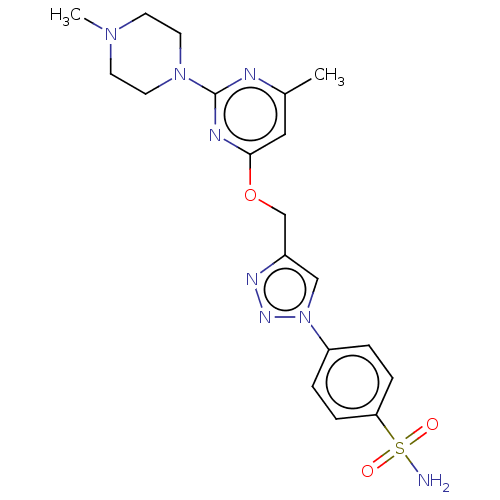
(CHEMBL4861411)Show SMILES CN1CCN(CC1)c1nc(C)cc(OCc2cn(nn2)-c2ccc(cc2)S(N)(=O)=O)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CA12 measured by stopped flow CO2 hydrase assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128249
BindingDB Entry DOI: 10.7270/Q2DR308N |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50252996
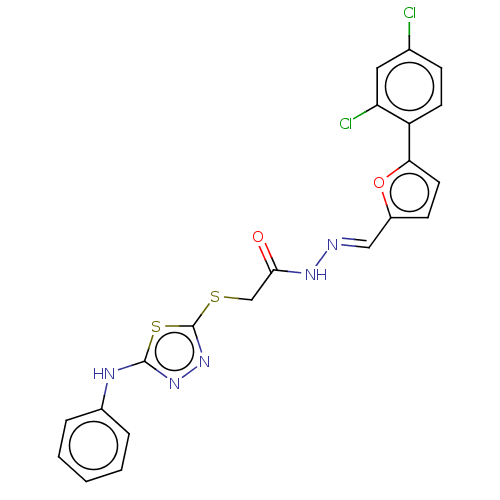
(CHEMBL4097972)Show SMILES Clc1ccc(-c2ccc(\C=N\NC(=O)CSc3nnc(Nc4ccccc4)s3)o2)c(Cl)c1 Show InChI InChI=1S/C21H15Cl2N5O2S2/c22-13-6-8-16(17(23)10-13)18-9-7-15(30-18)11-24-26-19(29)12-31-21-28-27-20(32-21)25-14-4-2-1-3-5-14/h1-11H,12H2,(H,25,27)(H,26,29)/b24-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr.
Curated by ChEMBL
| Assay Description
Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... |
Bioorg Med Chem 25: 3547-3554 (2017)
Article DOI: 10.1016/j.bmc.2017.05.005
BindingDB Entry DOI: 10.7270/Q28G8P41 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50155556

(2,3-dihydro-1H-indene-5-sulfonamide | CHEMBL364869...)Show InChI InChI=1S/C9H11NO2S/c10-13(11,12)9-5-4-7-2-1-3-8(7)6-9/h4-6H,1-3H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 by CO2 hydration assay |
Eur J Med Chem 43: 2853-60 (2008)
Article DOI: 10.1016/j.ejmech.2008.02.018
BindingDB Entry DOI: 10.7270/Q2Z03922 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222046
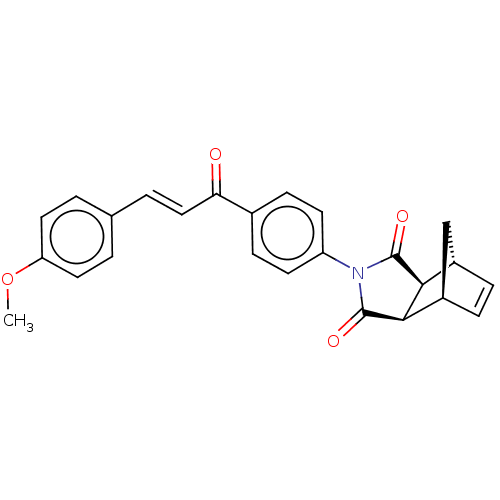
((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-methoxyphenyl)acryl...)Show SMILES COc1ccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)cc1 |r,c:24| Show InChI InChI=1S/C25H21NO4/c1-30-20-11-2-15(3-12-20)4-13-21(27)16-7-9-19(10-8-16)26-24(28)22-17-5-6-18(14-17)23(22)25(26)29/h2-13,17-18,22-23H,14H2,1H3/b13-4+/t17-,18+,22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222047
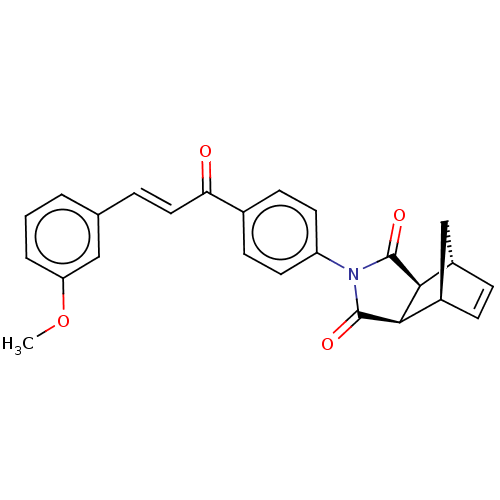
((3aR,4S,7R,7aS)-2-(4-((E)-3-(3-methoxyphenyl)acryl...)Show SMILES COc1cccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)c1 |r,c:25| Show InChI InChI=1S/C25H21NO4/c1-30-20-4-2-3-15(13-20)5-12-21(27)16-8-10-19(11-9-16)26-24(28)22-17-6-7-18(14-17)23(22)25(26)29/h2-13,17-18,22-23H,14H2,1H3/b12-5+/t17-,18+,22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222048
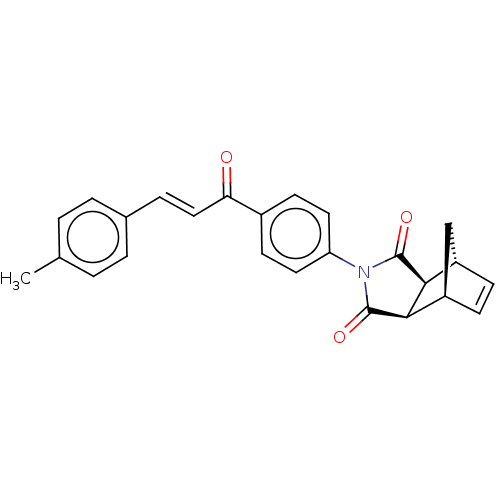
((3aR,4S,7R,7aS)-2-(4-((E)-3-(p-tolyl)acryloyl)phen...)Show SMILES Cc1ccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)cc1 |r,c:23| Show InChI InChI=1S/C25H21NO3/c1-15-2-4-16(5-3-15)6-13-21(27)17-9-11-20(12-10-17)26-24(28)22-18-7-8-19(14-18)23(22)25(26)29/h2-13,18-19,22-23H,14H2,1H3/b13-6+/t18-,19+,22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222049
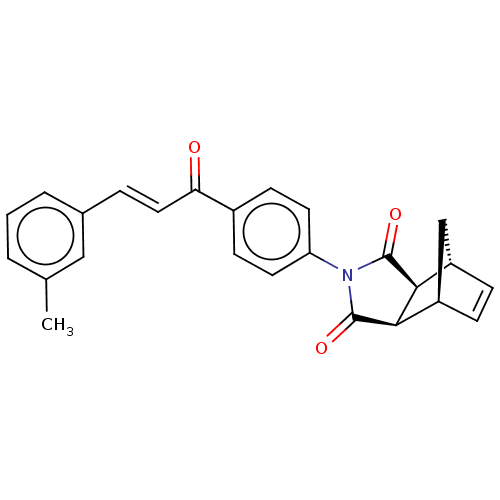
((3aR,4S,7R,7aS)-2-(4-((E)-3-(m-tolyl)acryloyl)phen...)Show SMILES Cc1cccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)c1 |r,c:24| Show InChI InChI=1S/C25H21NO3/c1-15-3-2-4-16(13-15)5-12-21(27)17-8-10-20(11-9-17)26-24(28)22-18-6-7-19(14-18)23(22)25(26)29/h2-13,18-19,22-23H,14H2,1H3/b12-5+/t18-,19+,22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222050
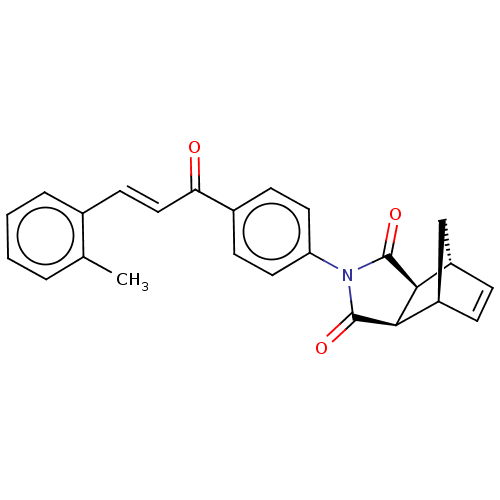
((3aR,4S,7R,7aS)-2-(4-((E)-3-(o-tolyl)acryloyl)phen...)Show SMILES Cc1ccccc1\C=C\C(=O)c1ccc(cc1)N1C(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@H]2C1=O |r,c:26| Show InChI InChI=1S/C25H21NO3/c1-15-4-2-3-5-16(15)10-13-21(27)17-8-11-20(12-9-17)26-24(28)22-18-6-7-19(14-18)23(22)25(26)29/h2-13,18-19,22-23H,14H2,1H3/b13-10+/t18-,19+,22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222051
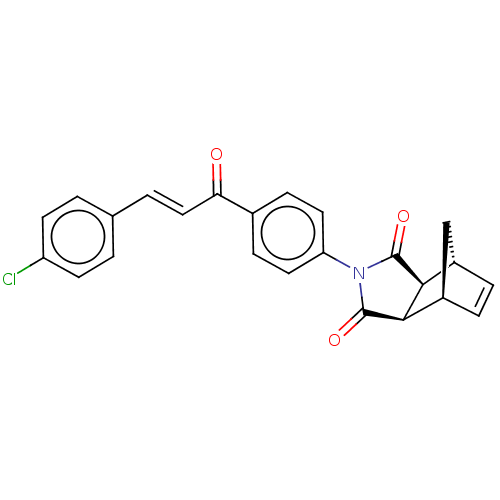
((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-chlorophenyl)acrylo...)Show SMILES Clc1ccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)cc1 |r,c:23| Show InChI InChI=1S/C24H18ClNO3/c25-18-8-1-14(2-9-18)3-12-20(27)15-6-10-19(11-7-15)26-23(28)21-16-4-5-17(13-16)22(21)24(26)29/h1-12,16-17,21-22H,13H2/b12-3+/t16-,17+,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222052
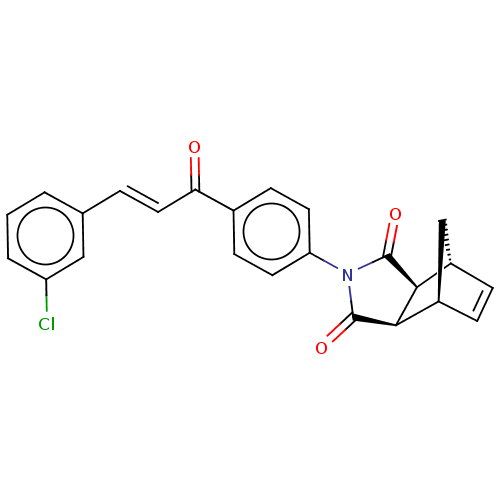
((3aR,4S,7R,7aS)-2-(4-((E)-3-(3-chlorophenyl)acrylo...)Show SMILES Clc1cccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)c1 |r,c:24| Show InChI InChI=1S/C24H18ClNO3/c25-18-3-1-2-14(12-18)4-11-20(27)15-7-9-19(10-8-15)26-23(28)21-16-5-6-17(13-16)22(21)24(26)29/h1-12,16-17,21-22H,13H2/b11-4+/t16-,17+,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222053
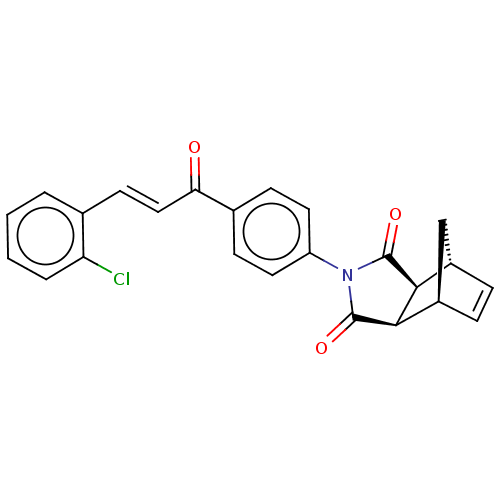
((3aR,4S,7R,7aS)-2-(4-((E)-3-(2-chlorophenyl)acrylo...)Show SMILES Clc1ccccc1\C=C\C(=O)c1ccc(cc1)N1C(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@H]2C1=O |r,c:26| Show InChI InChI=1S/C24H18ClNO3/c25-19-4-2-1-3-14(19)9-12-20(27)15-7-10-18(11-8-15)26-23(28)21-16-5-6-17(13-16)22(21)24(26)29/h1-12,16-17,21-22H,13H2/b12-9+/t16-,17+,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222054

((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-bromophenyl)acryloy...)Show SMILES Brc1ccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)cc1 |r,c:23| Show InChI InChI=1S/C24H18BrNO3/c25-18-8-1-14(2-9-18)3-12-20(27)15-6-10-19(11-7-15)26-23(28)21-16-4-5-17(13-16)22(21)24(26)29/h1-12,16-17,21-22H,13H2/b12-3+/t16-,17+,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222055
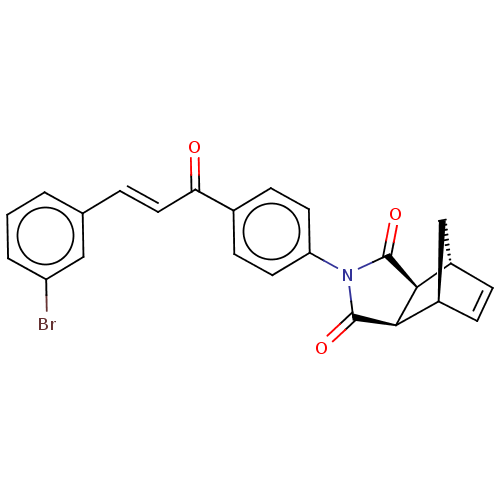
((3aR,4S,7R,7aS)-2-(4-((E)-3-(3-bromophenyl)acryloy...)Show SMILES Brc1cccc(\C=C\C(=O)c2ccc(cc2)N2C(=O)[C@H]3[C@@H]4C[C@@H](C=C4)[C@H]3C2=O)c1 |r,c:24| Show InChI InChI=1S/C24H18BrNO3/c25-18-3-1-2-14(12-18)4-11-20(27)15-7-9-19(10-8-15)26-23(28)21-16-5-6-17(13-16)22(21)24(26)29/h1-12,16-17,21-22H,13H2/b11-4+/t16-,17+,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222056
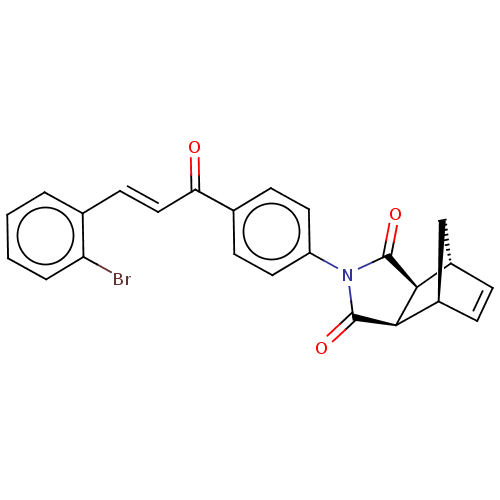
((3aR,4S,7R,7aS)-2-(4-((E)-3-(2-bromophenyl)acryloy...)Show SMILES Brc1ccccc1\C=C\C(=O)c1ccc(cc1)N1C(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@H]2C1=O |r,c:26| Show InChI InChI=1S/C24H18BrNO3/c25-19-4-2-1-3-14(19)9-12-20(27)15-7-10-18(11-8-15)26-23(28)21-16-5-6-17(13-16)22(21)24(26)29/h1-12,16-17,21-22H,13H2/b12-9+/t16-,17+,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222057
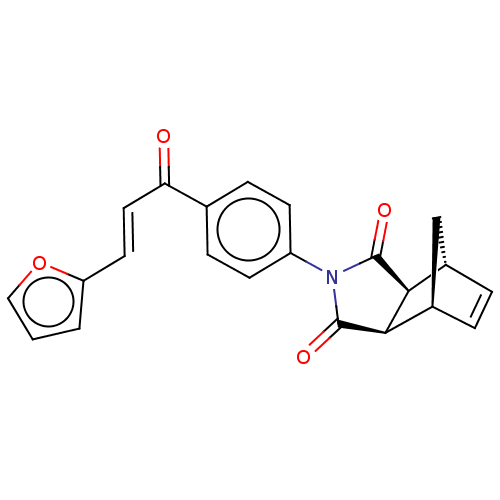
((3aR,4S,7R,7aS)-2-(4-((E)-3-(furan-2-yl)acryloyl)p...)Show SMILES O=C(\C=C\c1ccco1)c1ccc(cc1)N1C(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@H]2C1=O |r,c:24| Show InChI InChI=1S/C22H17NO4/c24-18(10-9-17-2-1-11-27-17)13-5-7-16(8-6-13)23-21(25)19-14-3-4-15(12-14)20(19)22(23)26/h1-11,14-15,19-20H,12H2/b10-9+/t14-,15+,19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222058
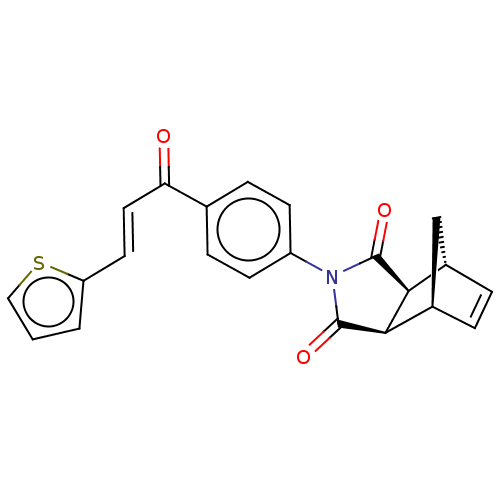
((3aR,4S,7R,7aS)-2-(4-((E)-3-(thiophen-2-yl)acryloy...)Show SMILES O=C(\C=C\c1cccs1)c1ccc(cc1)N1C(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@H]2C1=O |r,c:24| Show InChI InChI=1S/C22H17NO3S/c24-18(10-9-17-2-1-11-27-17)13-5-7-16(8-6-13)23-21(25)19-14-3-4-15(12-14)20(19)22(23)26/h1-11,14-15,19-20H,12H2/b10-9+/t14-,15+,19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222059
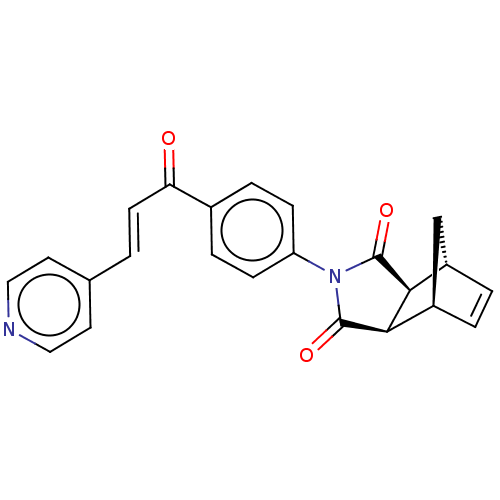
((3aR,4S,7R,7aS)-2-(4-((E)-3-(pyridin-4-yl)acryloyl...)Show SMILES O=C(\C=C\c1ccncc1)c1ccc(cc1)N1C(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@H]2C1=O |r,c:25| Show InChI InChI=1S/C23H18N2O3/c26-19(8-1-14-9-11-24-12-10-14)15-4-6-18(7-5-15)25-22(27)20-16-2-3-17(13-16)21(20)23(25)28/h1-12,16-17,20-21H,13H2/b8-1+/t16-,17+,20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM222056

((3aR,4S,7R,7aS)-2-(4-((E)-3-(2-bromophenyl)acryloy...)Show SMILES Brc1ccccc1\C=C\C(=O)c1ccc(cc1)N1C(=O)[C@H]2[C@@H]3C[C@@H](C=C3)[C@H]2C1=O |r,c:26| Show InChI InChI=1S/C24H18BrNO3/c25-19-4-2-1-3-14(19)9-12-20(27)15-7-10-18(11-8-15)26-23(28)21-16-5-6-17(13-16)22(21)24(26)29/h1-12,16-17,21-22H,13H2/b12-9+/t16-,17+,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.405 | -53.6 | 0.573 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University
| Assay Description
Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... |
Bioorg Chem 70: 118-125 (2017)
Article DOI: 10.1016/j.bioorg.2016.12.001
BindingDB Entry DOI: 10.7270/Q2CF9NZB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133394
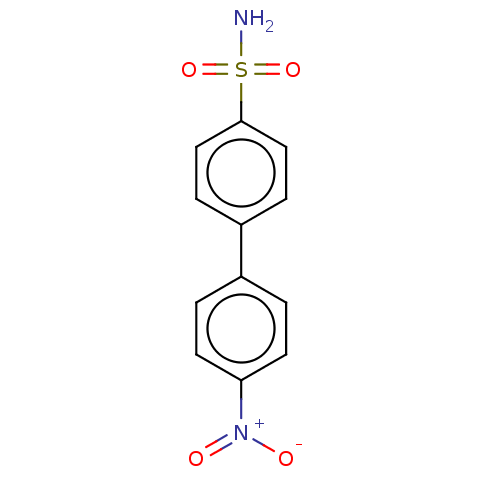
(CHEMBL3632830)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C12H10N2O4S/c13-19(17,18)12-7-3-10(4-8-12)9-1-5-11(6-2-9)14(15)16/h1-8H,(H2,13,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50133400
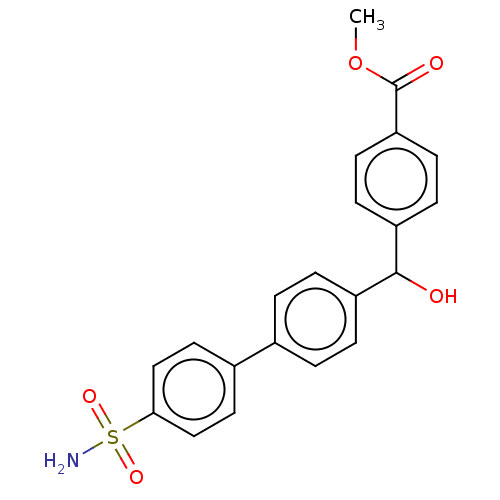
(CHEMBL3632836)Show SMILES COC(=O)c1ccc(cc1)C(O)c1ccc(cc1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H19NO5S/c1-27-21(24)18-8-6-17(7-9-18)20(23)16-4-2-14(3-5-16)15-10-12-19(13-11-15)28(22,25)26/h2-13,20,23H,1H3,(H2,22,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-14 assessed as CO2 hydration activity by stopped-flow method |
J Med Chem 58: 8564-72 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01144
BindingDB Entry DOI: 10.7270/Q2FF3V5V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50492476
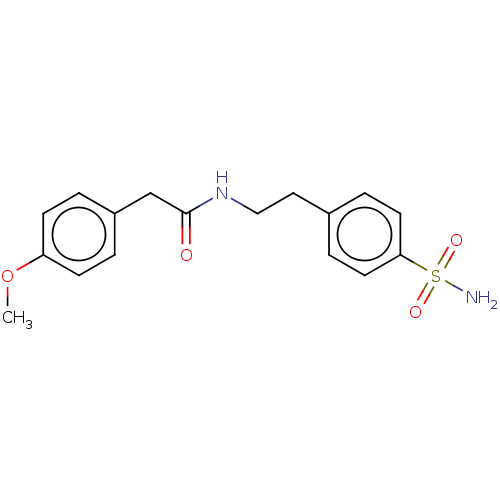
(CHEMBL1518649)Show InChI InChI=1S/C17H20N2O4S/c1-23-15-6-2-14(3-7-15)12-17(20)19-11-10-13-4-8-16(9-5-13)24(18,21)22/h2-9H,10-12H2,1H3,(H,19,20)(H2,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 5228-32 (2013)
Article DOI: 10.1016/j.bmc.2013.06.029
BindingDB Entry DOI: 10.7270/Q2CJ8HFM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data