

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP3 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM50056999 (CHEMBL56367 | nimesulide) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate preinc... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM25753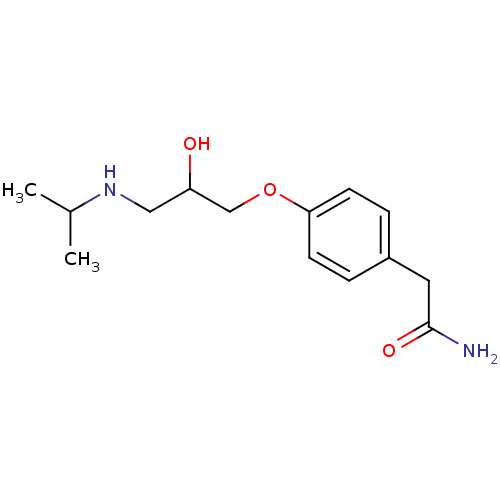 (2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM25756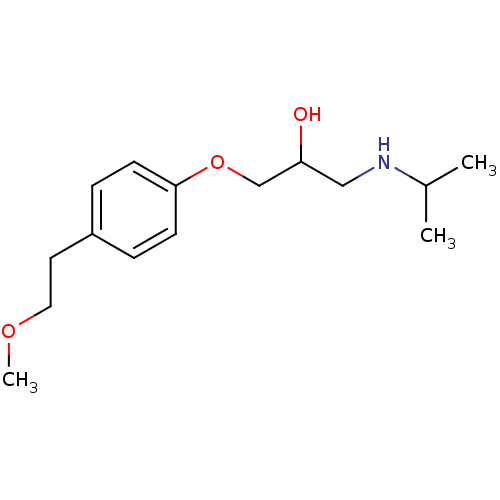 ((2R,3R)-2,3-dihydroxysuccinic acid;1-(isopropylami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM26197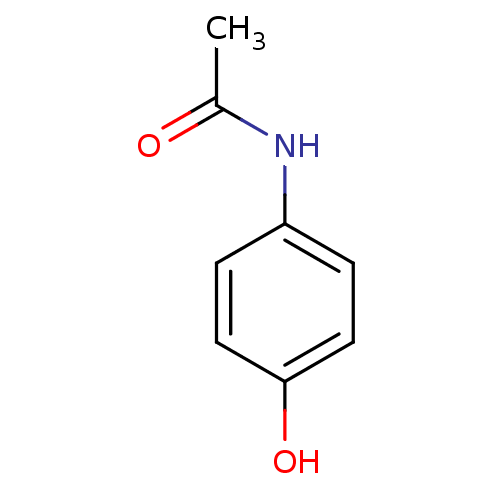 (CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM25758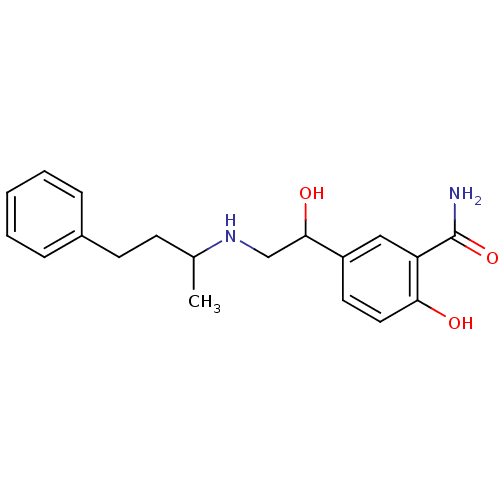 (2-hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amin...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM50174201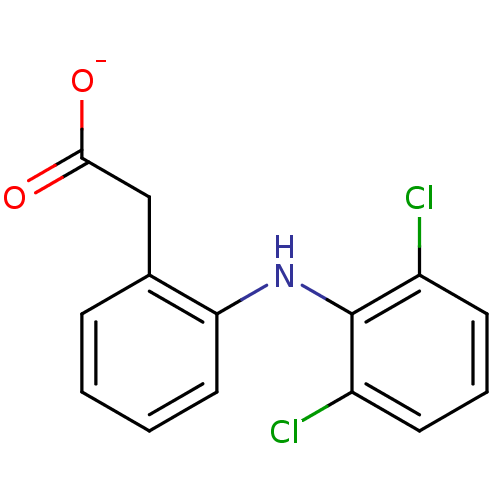 (ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595287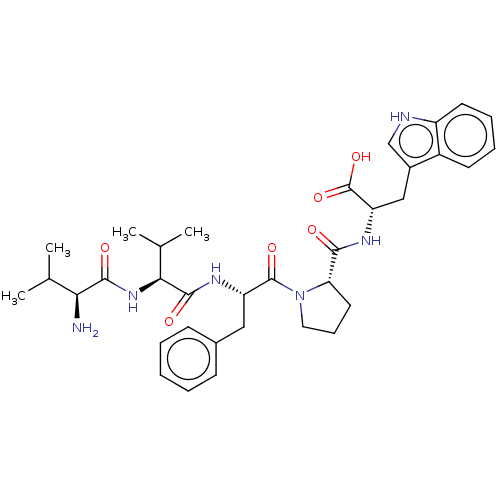 (CHEMBL5193958) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595285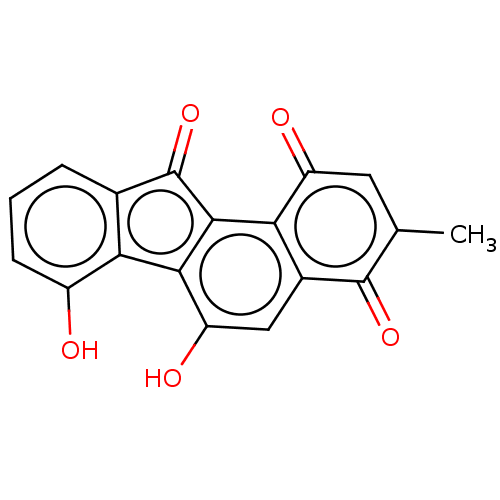 (CHEMBL5175079) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85267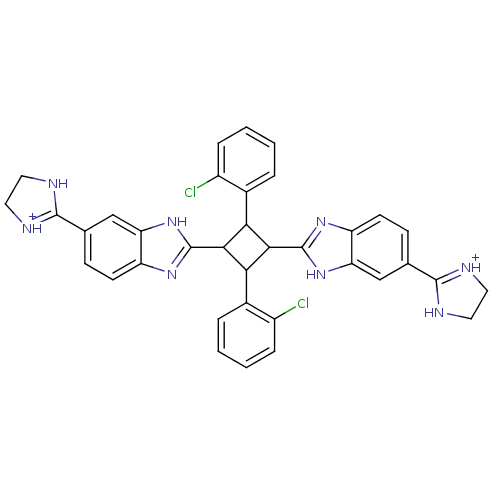 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85264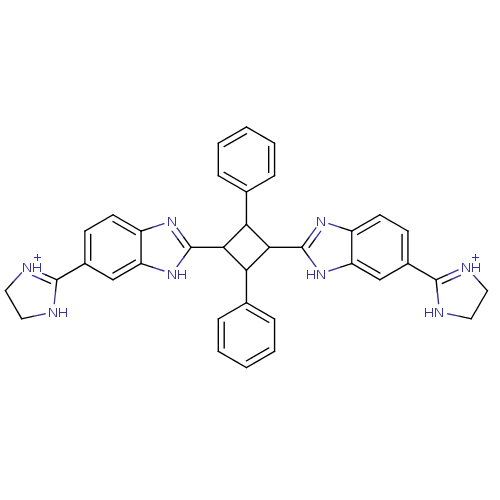 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | 200 | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595281 (CHEMBL5182137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85265 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85269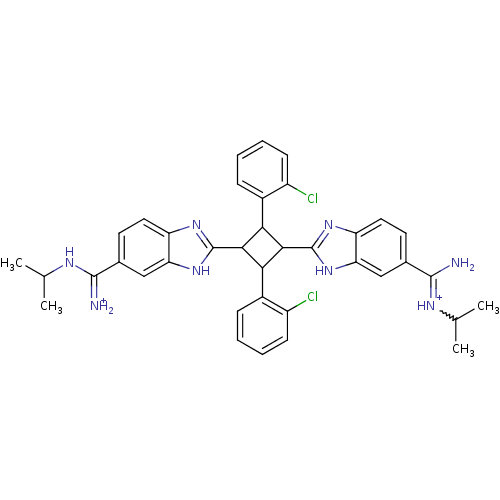 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85268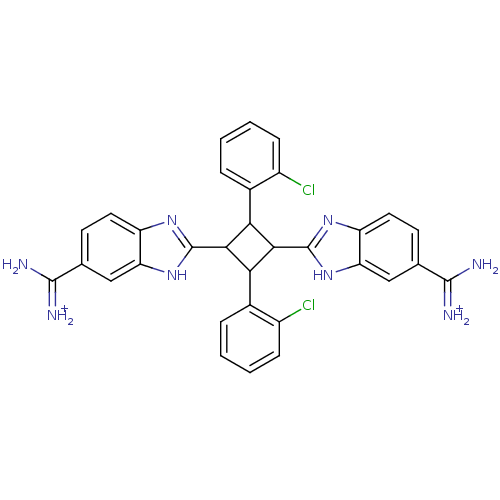 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595283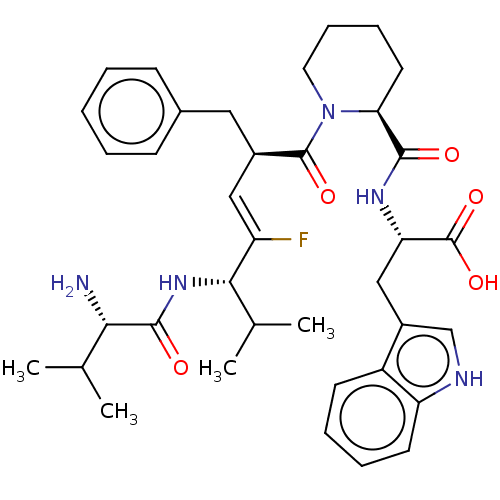 (CHEMBL5187966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85270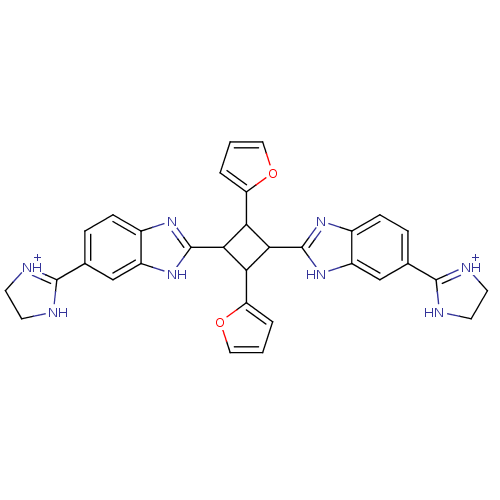 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595288 (CHEMBL5199877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85266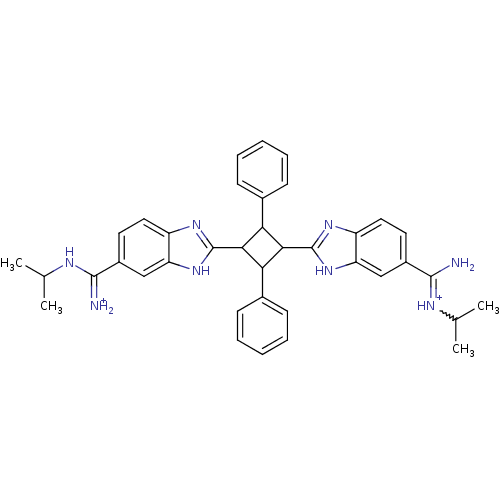 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85262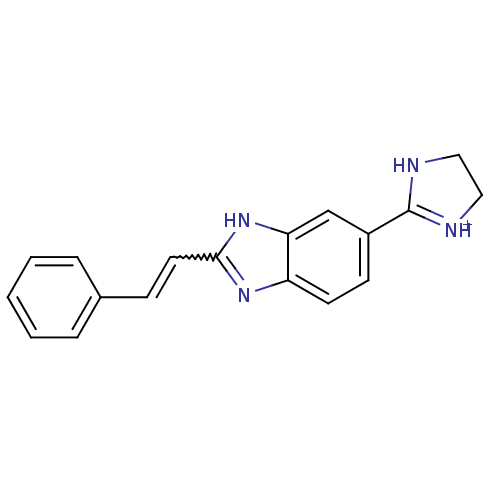 (Benzimidazole derivative, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85271 (2,4-Disubstituted-1,3-di-(5-amidino-2-benzoimidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85261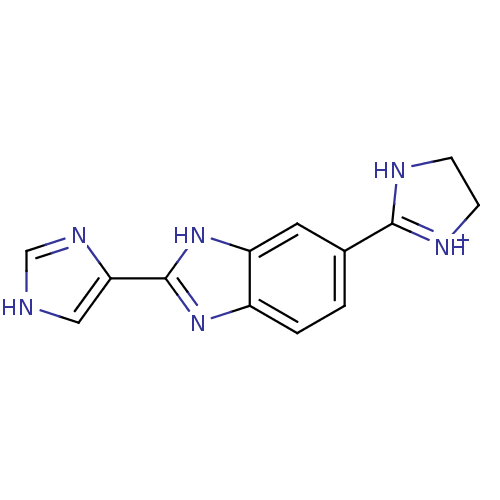 (Benzimidazole derivative, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595284 (CHEMBL5176913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595282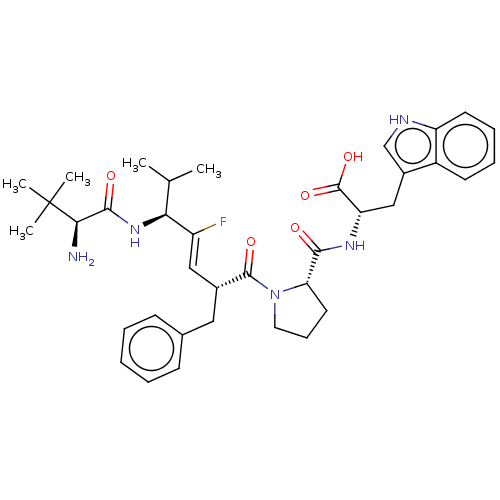 (CHEMBL5191808) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50049391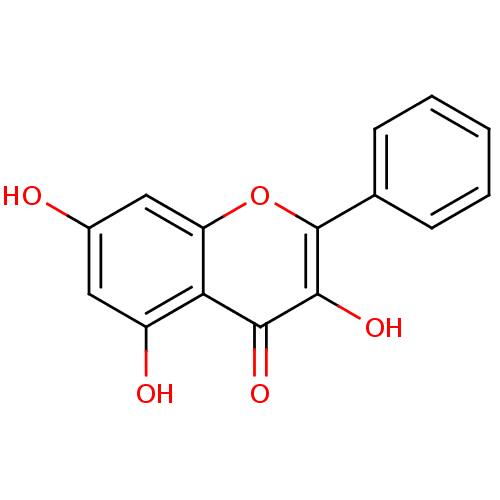 (3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM7457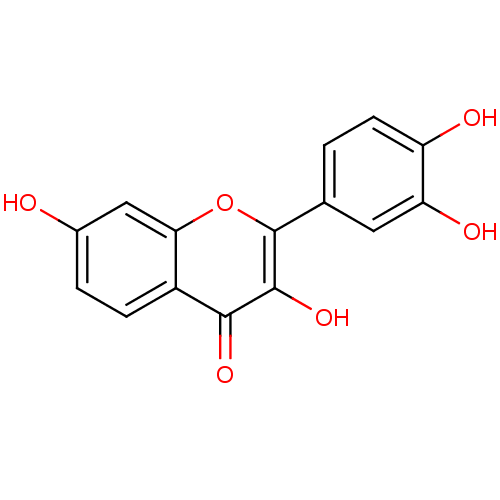 (2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187283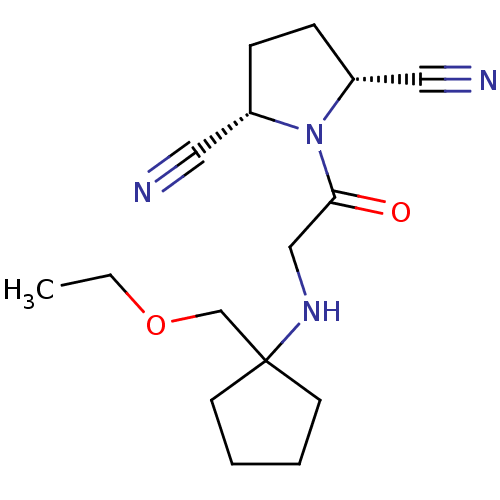 ((2R,5S)-1-(2-(1-(ethoxymethyl)cyclopentylamino)ace...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | J Med Chem 49: 3068-76 (2006) Article DOI: 10.1021/jm0600085 BindingDB Entry DOI: 10.7270/Q2BG2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50249479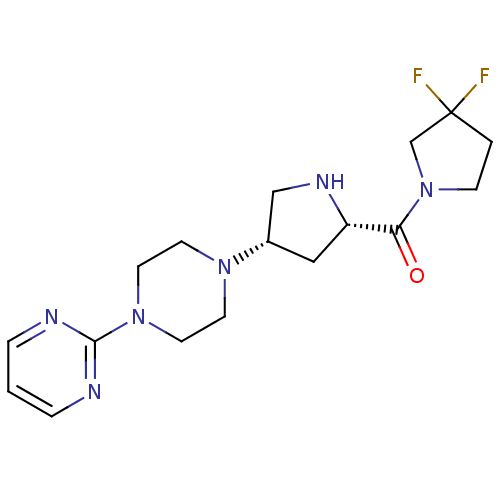 (2-(4-{(3S,5S)-5-[(3,3-difluoropyrrolidin-1-yl)carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of DPP3 in human erythrocytes | Bioorg Med Chem Lett 19: 1991-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.041 BindingDB Entry DOI: 10.7270/Q2FB52S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187258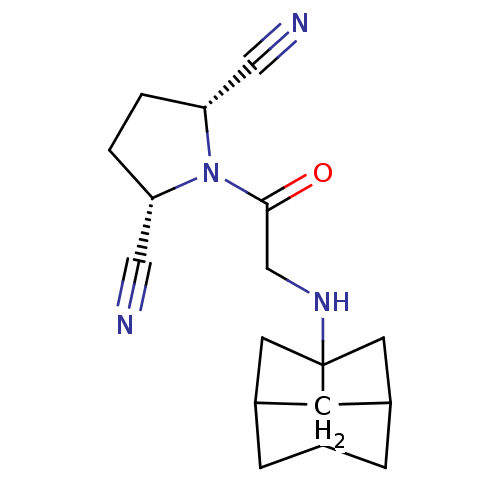 ((2S,5R)-1-[2-(hexahydro-2,5-methano-pentalen-3a-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | J Med Chem 49: 3068-76 (2006) Article DOI: 10.1021/jm0600085 BindingDB Entry DOI: 10.7270/Q2BG2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187261 ((2R,5S)-1-(2-(1-(butoxymethyl)cyclopentylamino)ace...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | J Med Chem 49: 3068-76 (2006) Article DOI: 10.1021/jm0600085 BindingDB Entry DOI: 10.7270/Q2BG2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187268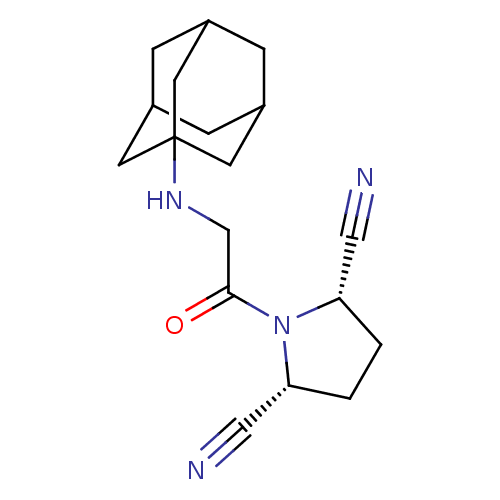 ((2S,5R)-1-[2-(adamantan-1-ylamino)-acetyl]-pyrroli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | J Med Chem 49: 3068-76 (2006) Article DOI: 10.1021/jm0600085 BindingDB Entry DOI: 10.7270/Q2BG2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187275 ((2R,5S)-1-(2-(1-(methoxymethyl)cyclopentylamino)ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | J Med Chem 49: 3068-76 (2006) Article DOI: 10.1021/jm0600085 BindingDB Entry DOI: 10.7270/Q2BG2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187263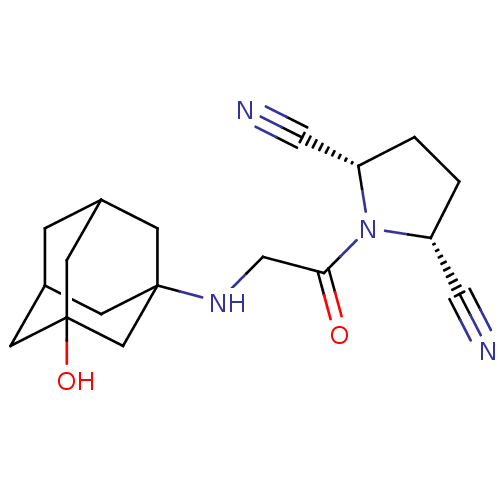 ((2S,5R)-1-[2-(3-hydroxy-adamantan-1-ylamino)-acety...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | J Med Chem 49: 3068-76 (2006) Article DOI: 10.1021/jm0600085 BindingDB Entry DOI: 10.7270/Q2BG2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187266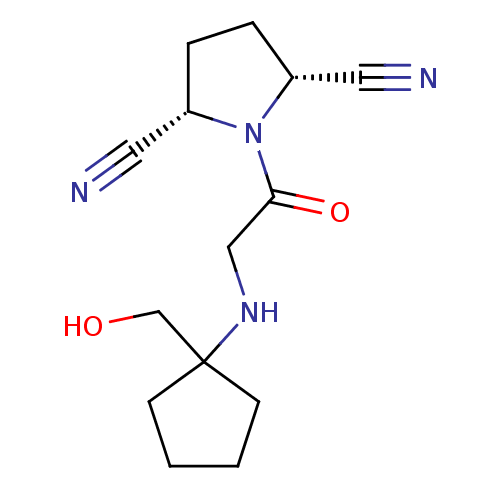 ((2R,5S)-1-(2-(1-(hydroxymethyl)cyclopentylamino)ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | J Med Chem 49: 3068-76 (2006) Article DOI: 10.1021/jm0600085 BindingDB Entry DOI: 10.7270/Q2BG2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50221073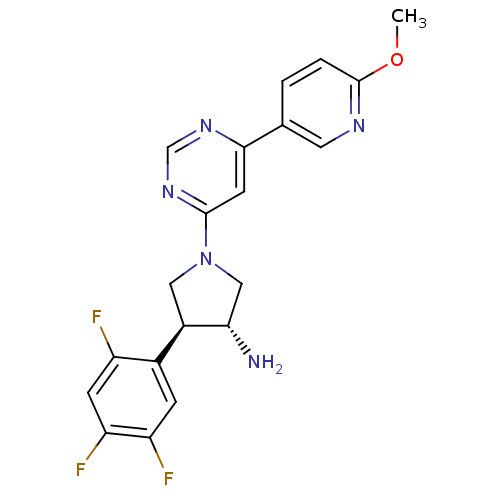 ((3R,4S)-1-(6-(6-methoxypyridin-3-yl)pyrimidin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of DPP3 | Bioorg Med Chem Lett 17: 5638-42 (2007) Article DOI: 10.1016/j.bmcl.2007.07.081 BindingDB Entry DOI: 10.7270/Q2319WRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.66E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85260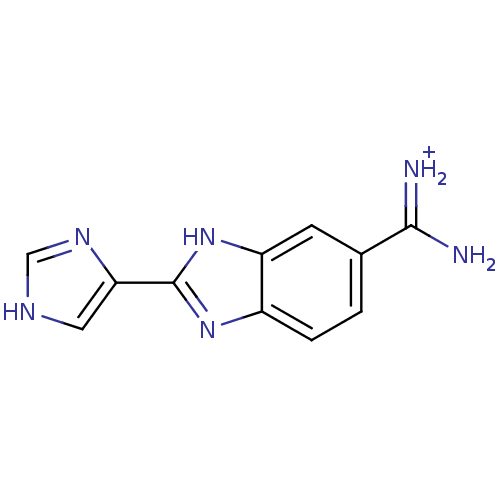 (Benzimidazole derivative, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187657 (3,6-dihydroxy-2-phenyl-4H-chromen-4-one | 3,6-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.64E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50595286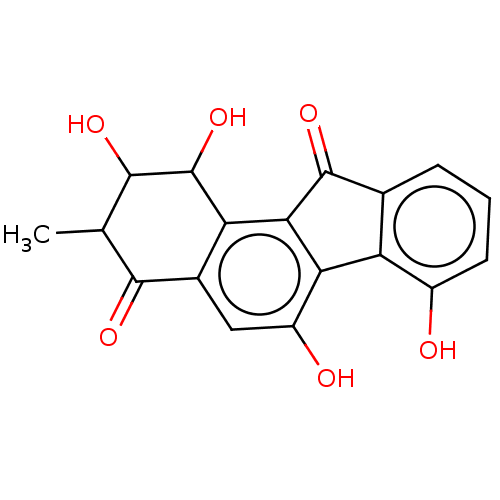 (CHEMBL5193133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116831 BindingDB Entry DOI: 10.7270/Q2183BJ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50081950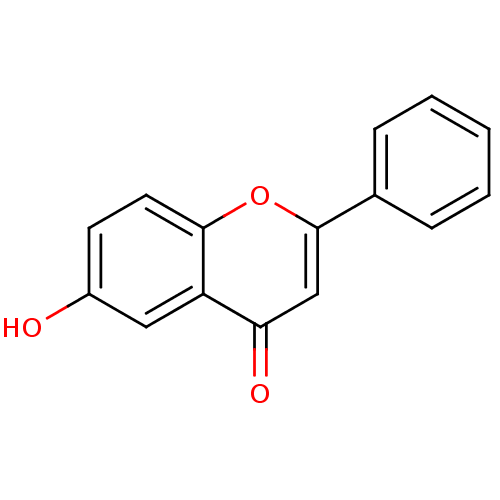 (6-Hydroxy-2-phenyl-chromen-4-one | 6-Hydroxyflavon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.22E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM26658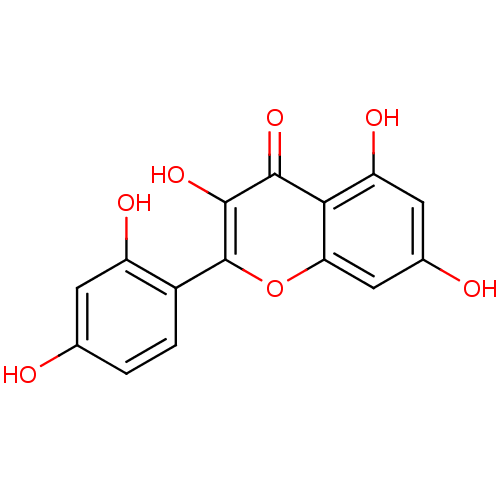 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM26660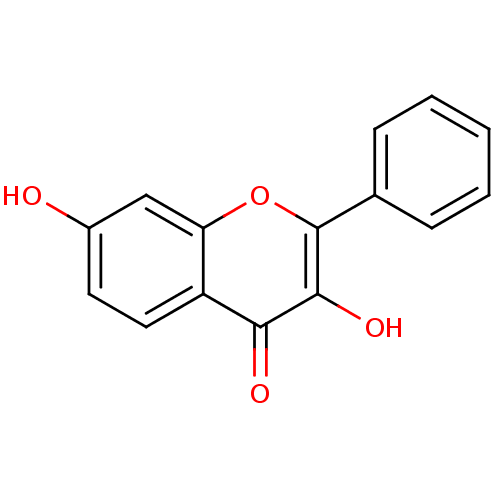 (3,7-dihydroxy-2-phenyl-4H-chromen-4-one | 3,7-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM85263 (Benzimidazole derivative, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
The Josip Juraj Strossmayer University | Assay Description The IC50 value is defined as the concentration of an inhibitor with caused 50% reduction of the enzyme activity under specific condition. The princi... | Bioorg Chem 35: 153-69 (2007) Article DOI: 10.1016/j.bioorg.2006.11.002 BindingDB Entry DOI: 10.7270/Q2NK3CKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM7461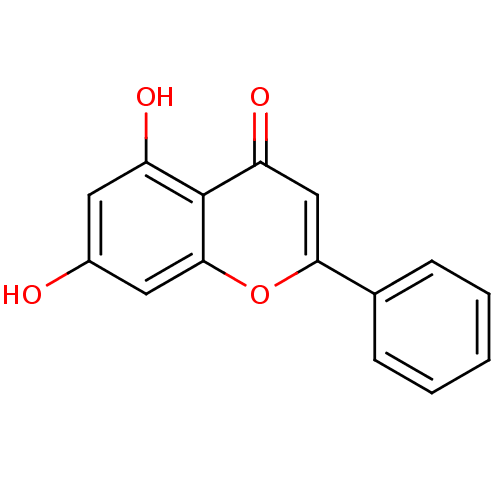 (5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50187668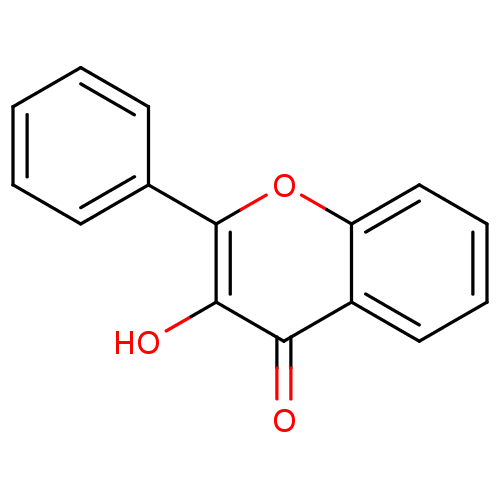 (3-Hydroxyflavone | 3-Hydroxyflavone (12) | 3-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Josip Juraj Strossmayer University of Osijek | Assay Description The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ... | Chem Biol Drug Des 89: 619-627 (2017) Article DOI: 10.1111/cbdd.12887 BindingDB Entry DOI: 10.7270/Q2X34WBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |