

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50110690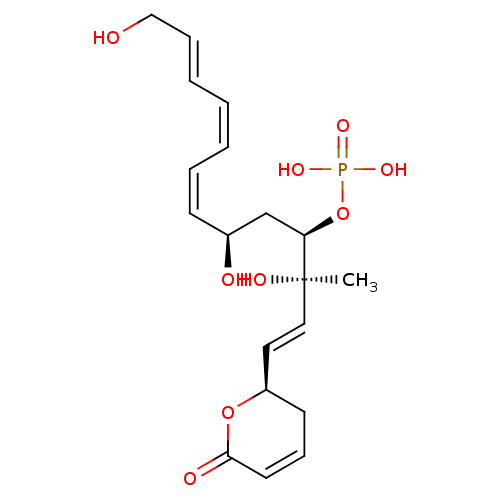 (CHEMBL17377 | FOSTRIECIN | Phosphoric acid mono-{3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50061067 (15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at NK2 receptor in human urinary bladder assessed as inhibition of [beta-Ala8]NKA(4-10)-induced tissue contractions | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50110681 (CHEMBL430266 | Calyculin-A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50408820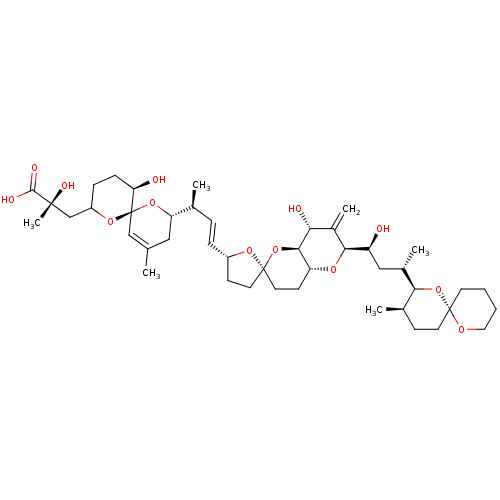 (CHEMBL5268133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at mouse histamine H4 receptor in SK-N-MC cells assessed as inhibition of forskolin-stimulated cAMP release preincubated for 10 m... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50408822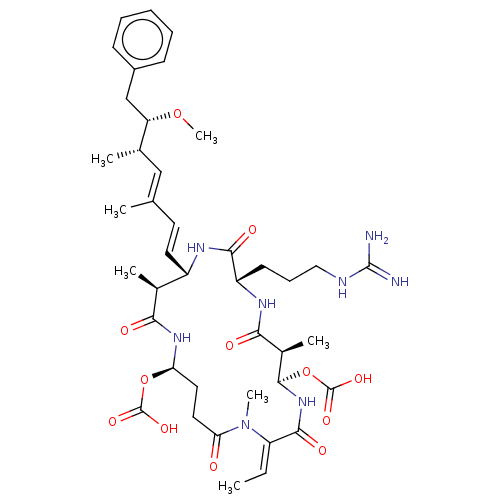 (CHEMBL5276260) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50366883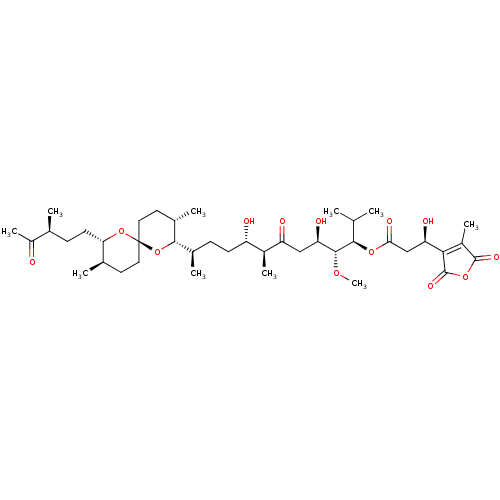 (TAUTOMYCIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at mouse histamine H4 receptor in SK-N-MC cells assessed as inhibition of forskolin-stimulated cAMP release preincubated for 10 m... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50408823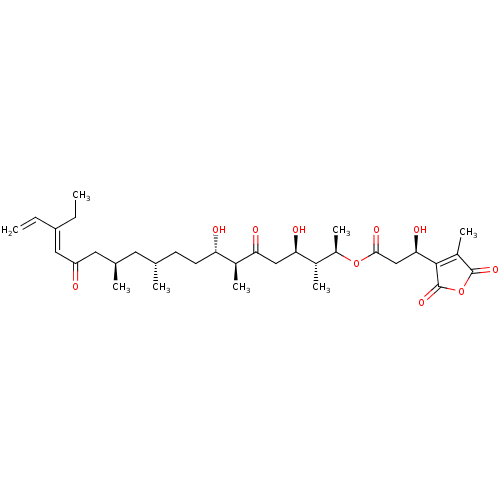 (CHEMBL5276593) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50090505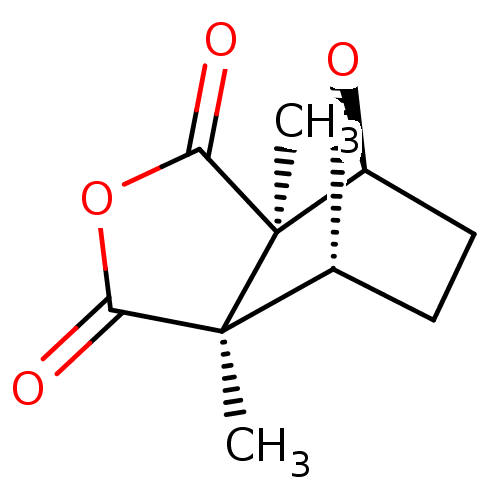 ((1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxa-tricyclo[5.2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50090505 ((1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxa-tricyclo[5.2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at rat H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after 6 hrs | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50607111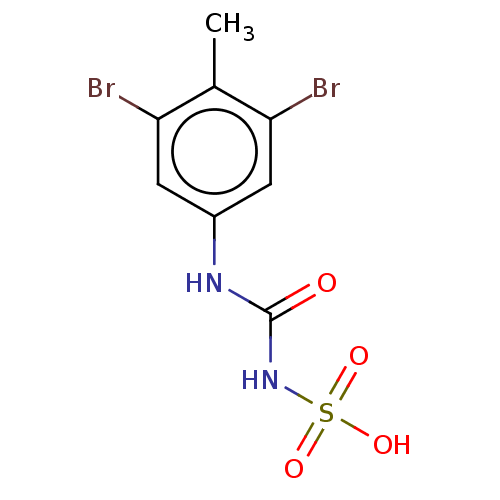 (CHEMBL5218807) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50087856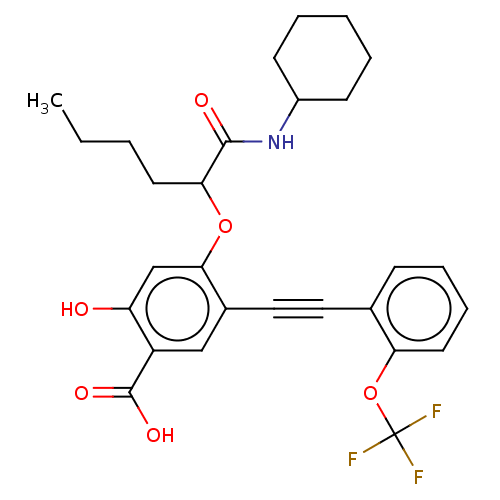 (CHEMBL3426913) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of PP5 (unknown origin) using pNPP as substrate at pH 7 at 25 degC by spectrophotometric analysis | Bioorg Med Chem 23: 2798-809 (2015) Article DOI: 10.1016/j.bmc.2015.03.066 BindingDB Entry DOI: 10.7270/Q2VD7168 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50112356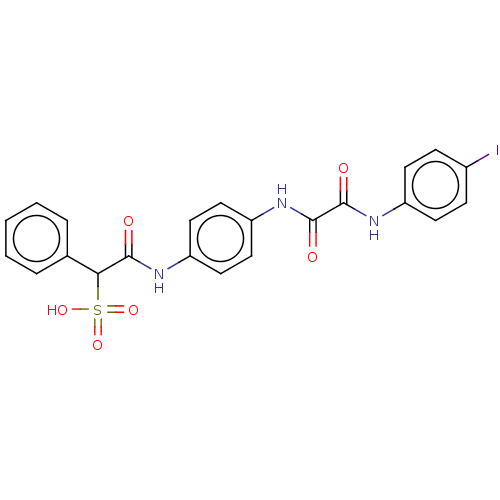 (CHEMBL3609373) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PP5 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50112357 (CHEMBL3609375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PP5 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50112358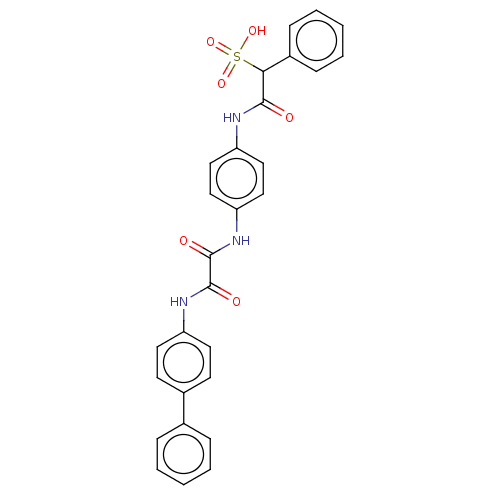 (CHEMBL3609374) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PP5 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50607110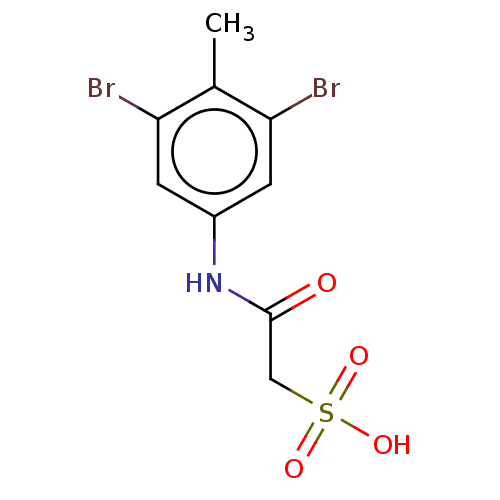 (CHEMBL5219519) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50420258 (CEFSULODIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PP5 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50558488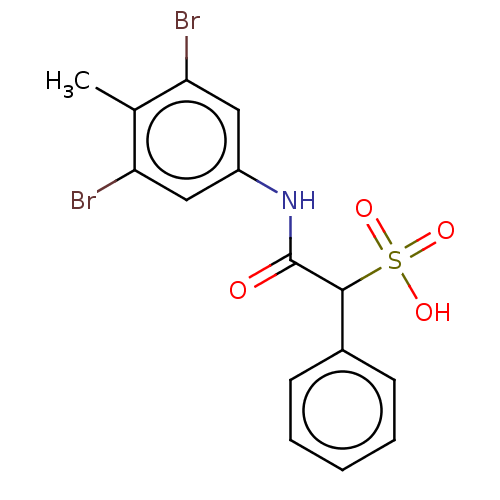 (CHEMBL4794972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM22319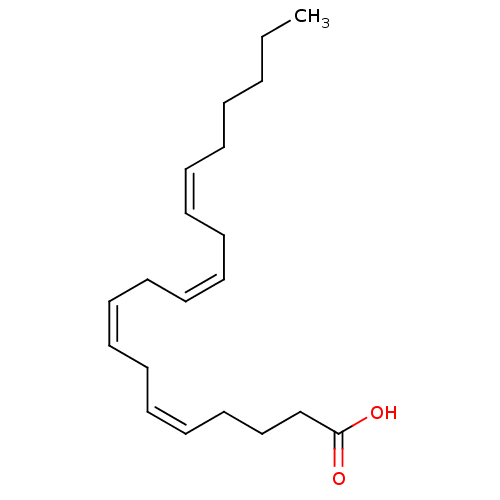 ((5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217376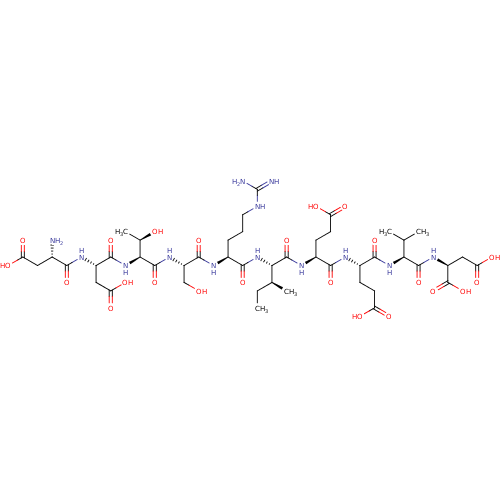 (DDTSRIEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 420 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217377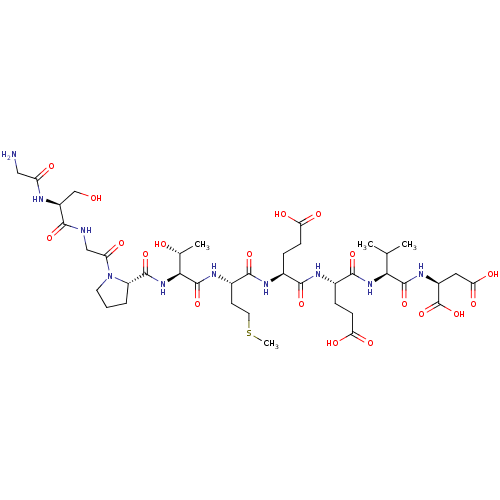 (GSGPTMEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 133 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217375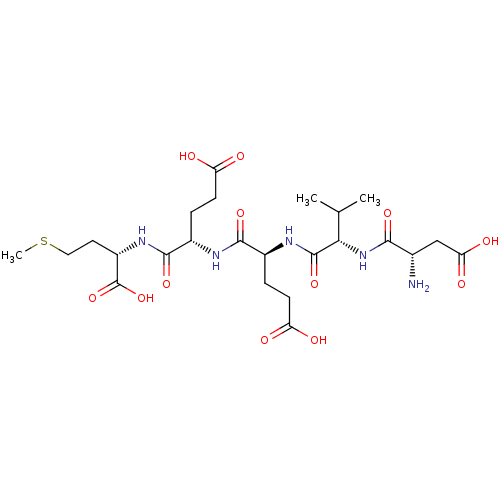 (DVEEM) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217372 (EDASRMEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 77 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217371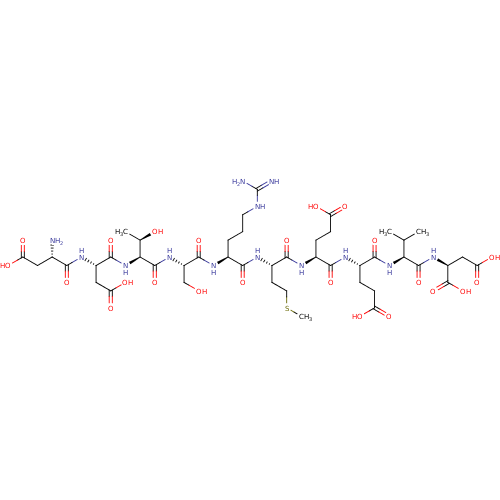 (DDTSRMEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 79 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217374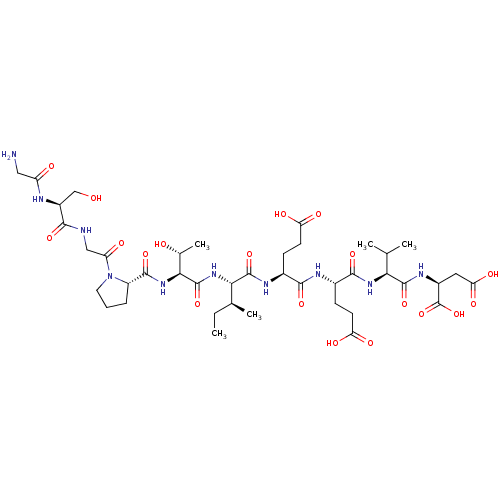 (GSGPTIEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217373 (SSGPTIEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM50408824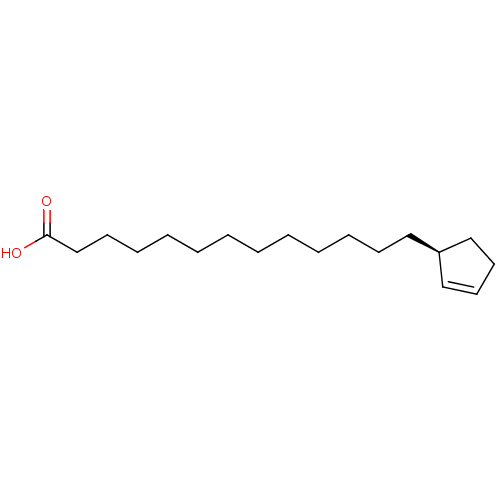 (CHAULMOOGRIC ACID | CHEBI:61391 | Chaulmoogric Aci...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217369 (MEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 140 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 5 (Homo sapiens (Human)) | BDBM217370 (IEEVD) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 426 | n/a | n/a | n/a | 7.4 | 25 |
University of Michigan | Assay Description All fluorescence polarization experiments were conducted in 384-well, black, low volume, round-bottomed plates (Corning) using a BioTeck Synergy 2 pl... | J Biol Chem 289: 2908-17 (2014) Article DOI: 10.1074/jbc.M113.519421 BindingDB Entry DOI: 10.7270/Q2GH9GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||