 Found 411 hits of ic50 for UniProtKB: P35367
Found 411 hits of ic50 for UniProtKB: P35367 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in HEK293 cells |
Bioorg Med Chem 24: 1793-810 (2016)
Article DOI: 10.1016/j.bmc.2016.03.006
BindingDB Entry DOI: 10.7270/Q2J67JS7 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor by radioligand displacement assay |
Bioorg Med Chem 21: 2764-71 (2013)
Article DOI: 10.1016/j.bmc.2013.03.016
BindingDB Entry DOI: 10.7270/Q2N87C5Q |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50240514
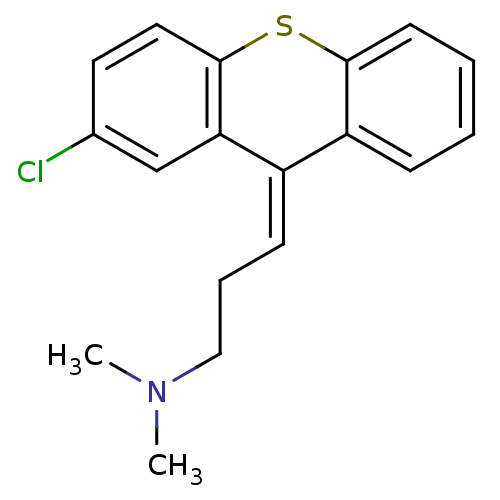
((3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dim...)Show InChI InChI=1S/C18H18ClNS/c1-20(2)11-5-7-14-15-6-3-4-8-17(15)21-18-10-9-13(19)12-16(14)18/h3-4,6-10,12H,5,11H2,1-2H3/b14-7- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Antagonist activity at H1 receptor in human HeLa cells assessed as inhibition of histamine-induced Ca2+ release by using fura-2AM-based fluorescence ... |
J Med Chem 55: 7054-60 (2012)
Article DOI: 10.1021/jm300671m
BindingDB Entry DOI: 10.7270/Q2FN17BZ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50112349
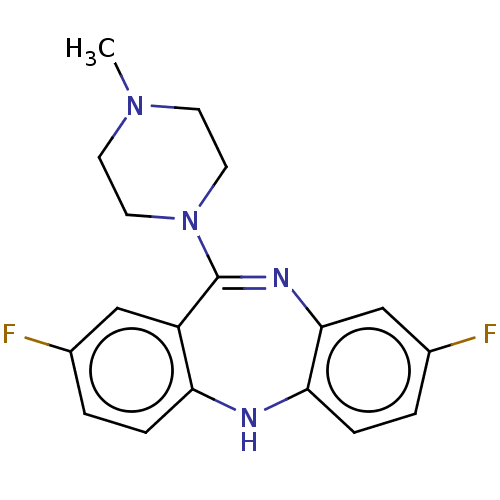
(CHEMBL3609328)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2Nc2ccc(F)cc12 |t:8| Show InChI InChI=1S/C18H18F2N4/c1-23-6-8-24(9-7-23)18-14-10-12(19)2-4-15(14)21-16-5-3-13(20)11-17(16)22-18/h2-5,10-11,21H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human histamine H1 receptor |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor (unknown origin) |
J Med Chem 58: 2584-608 (2015)
Article DOI: 10.1021/jm501535r
BindingDB Entry DOI: 10.7270/Q2JM2CBK |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM94597
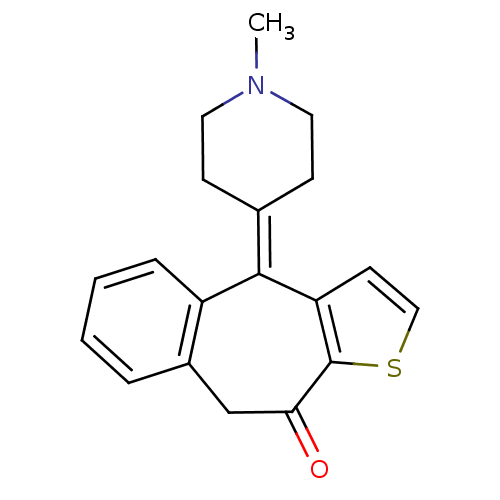
((Z)-2-butenedioate;10-(1-methyl-4-piperidinylidene...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccsc2-[#6](=O)-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H19NOS/c1-20-9-6-13(7-10-20)18-15-5-3-2-4-14(15)12-17(21)19-16(18)8-11-22-19/h2-5,8,11H,6-7,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Phrama. Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHOK1 cells by scintillation counting |
Bioorg Med Chem Lett 19: 2766-71 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.124
BindingDB Entry DOI: 10.7270/Q2GM87BK |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50240514

((3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dim...)Show InChI InChI=1S/C18H18ClNS/c1-20(2)11-5-7-14-15-6-3-4-8-17(15)21-18-10-9-13(19)12-16(14)18/h3-4,6-10,12H,5,11H2,1-2H3/b14-7- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Antagonist activity at H1 receptor in human HeLa cells assessed as inhibition of histamine-induced Ca2+ release by using fura-2AM-based fluorescence ... |
J Med Chem 55: 7054-60 (2012)
Article DOI: 10.1021/jm300671m
BindingDB Entry DOI: 10.7270/Q2FN17BZ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50002087
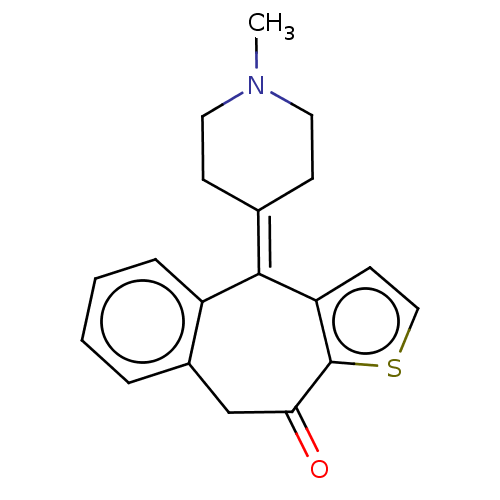
(4-(1-Methyl-piperidin-4-ylidene)-4,9-dihydro-1-thi...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccsc2-[#6](=O)-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H19NOS/c1-20-9-6-13(7-10-20)18-15-5-3-2-4-14(15)12-17(21)19-16(18)8-11-22-19/h2-5,8,11H,6-7,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 3005-21 (2011)
Article DOI: 10.1016/j.bmc.2011.03.003
BindingDB Entry DOI: 10.7270/Q23T9HJM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403992
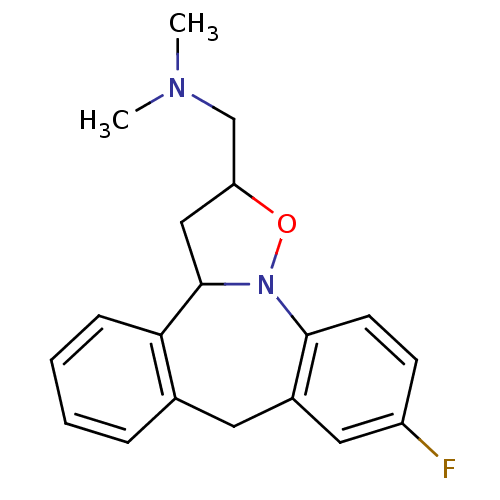
(CHEMBL84081)Show InChI InChI=1S/C19H21FN2O/c1-21(2)12-16-11-19-17-6-4-3-5-13(17)9-14-10-15(20)7-8-18(14)22(19)23-16/h3-8,10,16,19H,9,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant H1 receptor expressed in HEK293 cells measured after 60 mins by scintillation counting method |
Bioorg Med Chem 25: 471-482 (2017)
Article DOI: 10.1016/j.bmc.2016.11.014
BindingDB Entry DOI: 10.7270/Q2CF9S3S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403991
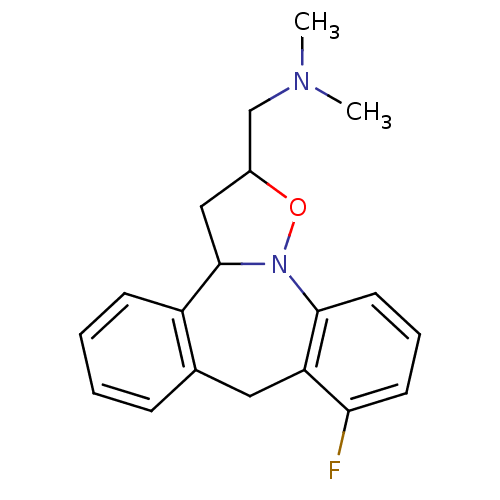
(CHEMBL315834)Show InChI InChI=1S/C19H21FN2O/c1-21(2)12-14-11-19-15-7-4-3-6-13(15)10-16-17(20)8-5-9-18(16)22(19)23-14/h3-9,14,19H,10-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50073179
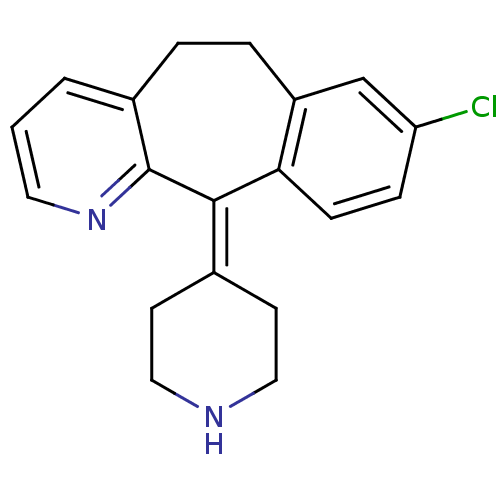
(8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)c1 Show InChI InChI=1S/C19H19ClN2/c20-16-5-6-17-15(12-16)4-3-14-2-1-9-22-19(14)18(17)13-7-10-21-11-8-13/h1-2,5-6,9,12,21H,3-4,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor in HEK293 cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403975
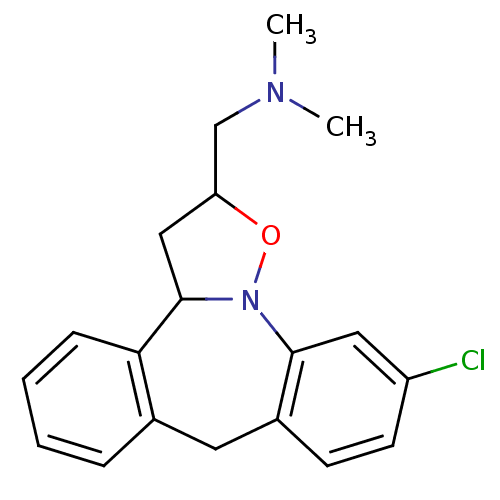
(CHEMBL315772)Show InChI InChI=1S/C19H21ClN2O/c1-21(2)12-16-11-19-17-6-4-3-5-13(17)9-14-7-8-15(20)10-18(14)22(19)23-16/h3-8,10,16,19H,9,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity against Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3573-7 (2002)
BindingDB Entry DOI: 10.7270/Q26M384X |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403986

(CHEMBL82966)Show InChI InChI=1S/C19H21FN2O/c1-21(2)12-14-11-19-15-7-5-8-17(20)16(15)10-13-6-3-4-9-18(13)22(19)23-14/h3-9,14,19H,10-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403975

(CHEMBL315772)Show InChI InChI=1S/C19H21ClN2O/c1-21(2)12-16-11-19-17-6-4-3-5-13(17)9-14-7-8-15(20)10-18(14)22(19)23-16/h3-8,10,16,19H,9,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403974
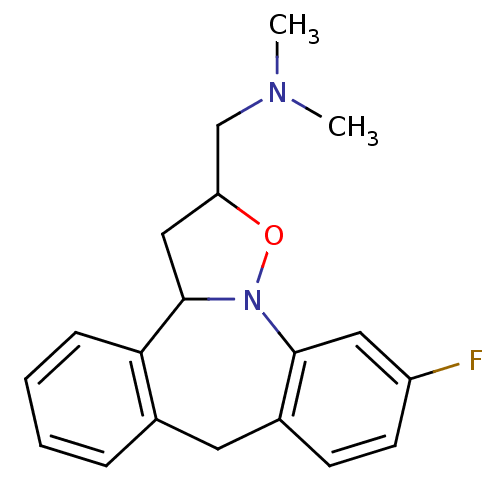
(CHEMBL84931)Show InChI InChI=1S/C19H21FN2O/c1-21(2)12-16-11-19-17-6-4-3-5-13(17)9-14-7-8-15(20)10-18(14)22(19)23-16/h3-8,10,16,19H,9,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403972
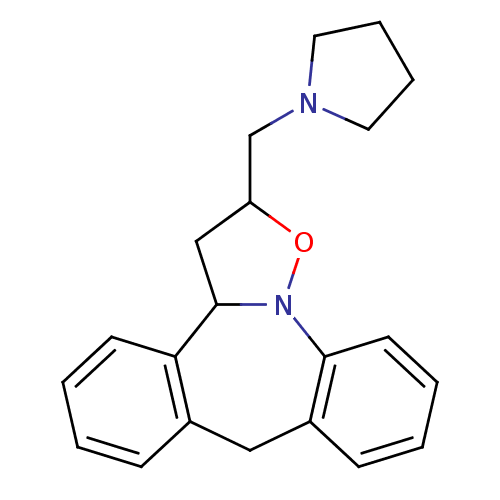
(CHEMBL316438)Show InChI InChI=1S/C21H24N2O/c1-3-9-19-16(7-1)13-17-8-2-4-10-20(17)23-21(19)14-18(24-23)15-22-11-5-6-12-22/h1-4,7-10,18,21H,5-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned Histamine H1 receptor expressed in CHO cells using [3H]pyrilamine as radioligand |
Bioorg Med Chem Lett 12: 243-8 (2001)
Article DOI: 10.1016/s0960-894x(01)00721-1
BindingDB Entry DOI: 10.7270/Q23J3F4C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529278
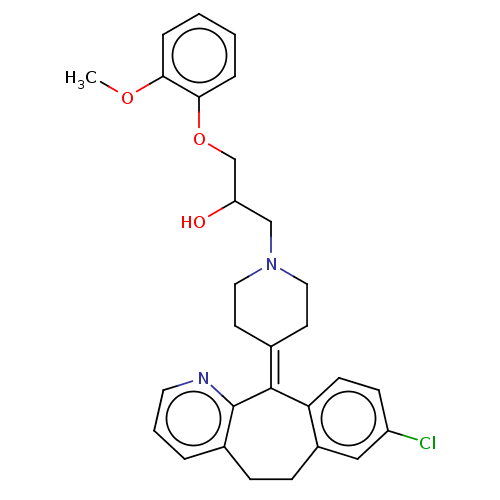
(CHEMBL4446961)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50175494
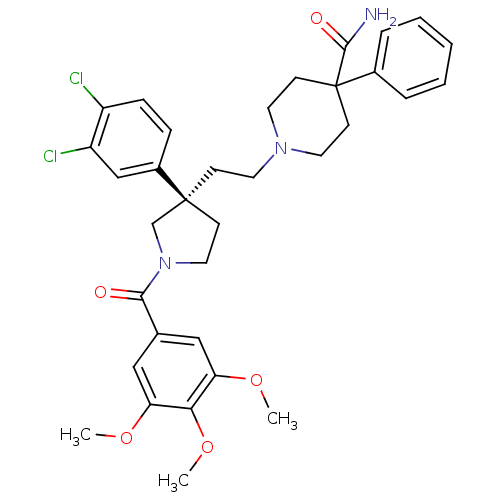
(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H39Cl2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adamed Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant H1 receptor assessed as inhibition of histamine-induced intracellular Ca2+ release |
J Med Chem 57: 4543-57 (2014)
Article DOI: 10.1021/jm401895u
BindingDB Entry DOI: 10.7270/Q2N29ZHX |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Displacement of [3H]Pyrilamine from human recombinant histamine H1 receptor expressed in CHOK1 cells after 3 hrs |
Bioorg Med Chem Lett 23: 1834-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.025
BindingDB Entry DOI: 10.7270/Q2P55PWC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297865

(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES CN(C1CCOCC1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C25H31FN4O/c1-28(22-12-16-31-17-13-22)21-10-14-29(15-11-21)25-27-23-4-2-3-5-24(23)30(25)18-19-6-8-20(26)9-7-19/h2-9,21-22H,10-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM31005
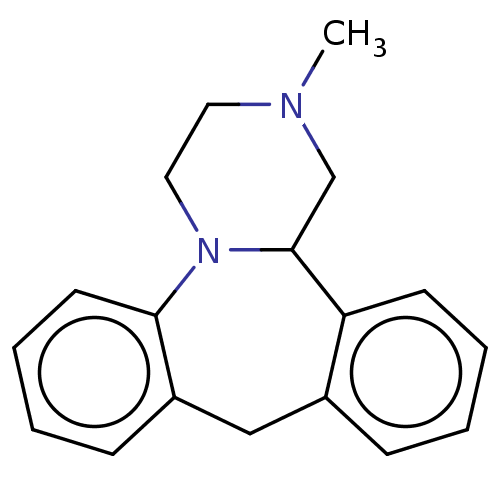
(2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyraz...)Show InChI InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned Histamine H1 receptor expressed in CHO cells using [3H]pyrilamine as radioligand |
Bioorg Med Chem Lett 12: 243-8 (2001)
Article DOI: 10.1016/s0960-894x(01)00721-1
BindingDB Entry DOI: 10.7270/Q23J3F4C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50172414
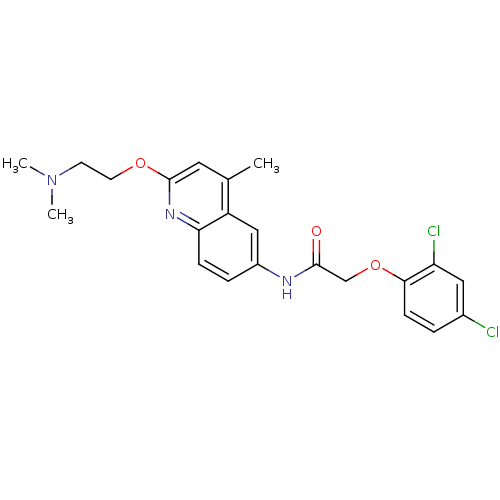
(2-(2,4-Dichloro-phenoxy)-N-[2-(2-dimethylamino-eth...)Show SMILES CN(C)CCOc1cc(C)c2cc(NC(=O)COc3ccc(Cl)cc3Cl)ccc2n1 Show InChI InChI=1S/C22H23Cl2N3O3/c1-14-10-22(29-9-8-27(2)3)26-19-6-5-16(12-17(14)19)25-21(28)13-30-20-7-4-15(23)11-18(20)24/h4-7,10-12H,8-9,13H2,1-3H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Inhibitory concentration against histamine H1 receptor |
J Med Chem 48: 5684-97 (2005)
Article DOI: 10.1021/jm050103y
BindingDB Entry DOI: 10.7270/Q2H41QZ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529267

(CHEMBL4591702)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6@H](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 |r| Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403983

(CHEMBL314885)Show InChI InChI=1S/C19H21FN2O/c1-21(2)12-16-11-19-17-10-15(20)8-7-13(17)9-14-5-3-4-6-18(14)22(19)23-16/h3-8,10,16,19H,9,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50036935
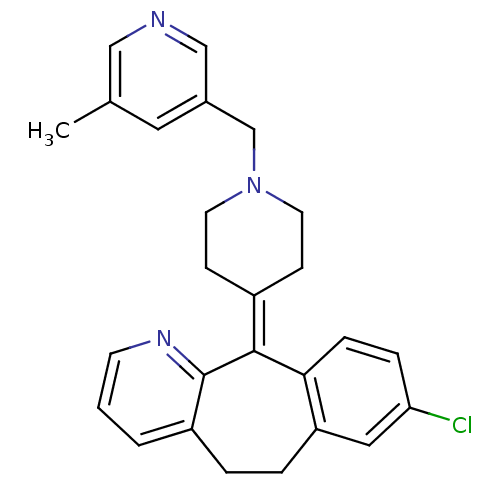
(8-Chloro-11-[1-(5-methyl-pyridin-3-ylmethyl)-piper...)Show SMILES [#6]-c1cncc(-[#6]-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#6]-[#6]-c3cccnc-23)c1 Show InChI InChI=1S/C26H26ClN3/c1-18-13-19(16-28-15-18)17-30-11-8-20(9-12-30)25-24-7-6-23(27)14-22(24)5-4-21-3-2-10-29-26(21)25/h2-3,6-7,10,13-16H,4-5,8-9,11-12,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297868
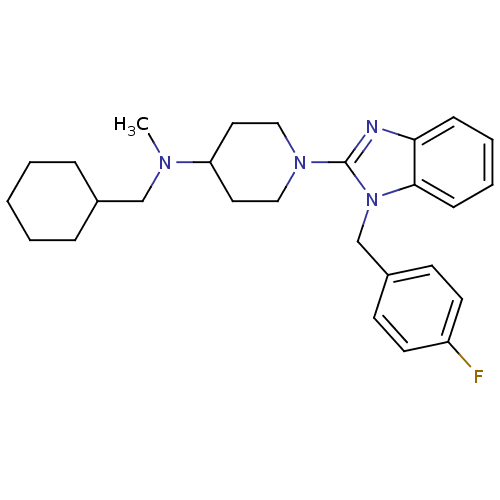
(CHEMBL549598 | N-(cyclohexylmethyl)-1-(1-(4-fluoro...)Show SMILES CN(CC1CCCCC1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C27H35FN4/c1-30(19-21-7-3-2-4-8-21)24-15-17-31(18-16-24)27-29-25-9-5-6-10-26(25)32(27)20-22-11-13-23(28)14-12-22/h5-6,9-14,21,24H,2-4,7-8,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290703

(4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoyl)-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(cc2)C(O)=O)(C1)c1ccccc1 Show InChI InChI=1S/C43H46N4O7/c1-52-36-25-32(26-37(53-2)39(36)54-3)41(49)46-24-20-43(28-46,33-9-5-4-6-10-33)19-23-45-21-17-30(18-22-45)38(48)40-44-34-11-7-8-12-35(34)47(40)27-29-13-15-31(16-14-29)42(50)51/h4-16,25-26,30H,17-24,27-28H2,1-3H3,(H,50,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529266
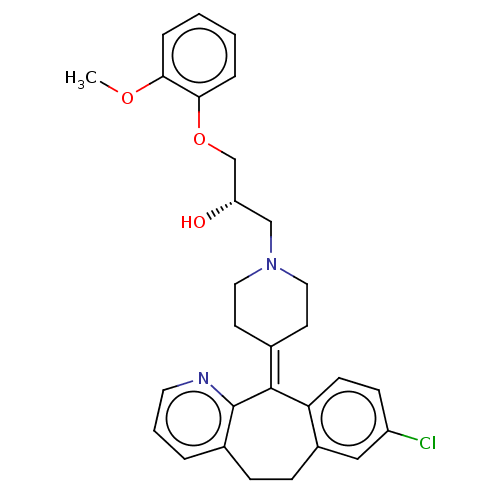
(CHEMBL4467816)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6@@H](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 |r| Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290742
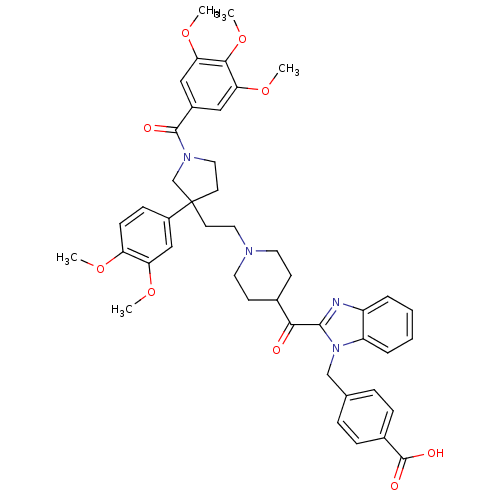
(4-[2-(1-{2-[3-(3,4-Dimethoxy-phenyl)-1-(3,4,5-trim...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccc(cc2)C(O)=O)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C45H50N4O9/c1-54-36-15-14-33(26-37(36)55-2)45(19-23-48(28-45)43(51)32-24-38(56-3)41(58-5)39(25-32)57-4)18-22-47-20-16-30(17-21-47)40(50)42-46-34-8-6-7-9-35(34)49(42)27-29-10-12-31(13-11-29)44(52)53/h6-15,24-26,30H,16-23,27-28H2,1-5H3,(H,52,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529273

(CHEMBL4556553)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1F)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H28ClFN2O2/c29-22-9-10-24-21(16-22)8-7-20-4-3-13-31-28(20)27(24)19-11-14-32(15-12-19)17-23(33)18-34-26-6-2-1-5-25(26)30/h1-6,9-10,13,16,23,33H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290746

(4-[2-(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2CCCC(O)=O)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C39H44Cl2N4O7/c1-50-32-21-26(22-33(51-2)36(32)52-3)38(49)44-20-15-39(24-44,27-10-11-28(40)29(41)23-27)14-19-43-17-12-25(13-18-43)35(48)37-42-30-7-4-5-8-31(30)45(37)16-6-9-34(46)47/h4-5,7-8,10-11,21-23,25H,6,9,12-20,24H2,1-3H3,(H,46,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290743

(2-{4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2CCCCOc2ccccc2C(O)=O)(C1)c1ccccc1 Show InChI InChI=1S/C46H52N4O8/c1-55-39-29-33(30-40(56-2)42(39)57-3)44(52)49-27-22-46(31-49,34-13-5-4-6-14-34)21-26-48-24-19-32(20-25-48)41(51)43-47-36-16-8-9-17-37(36)50(43)23-11-12-28-58-38-18-10-7-15-35(38)45(53)54/h4-10,13-18,29-30,32H,11-12,19-28,31H2,1-3H3,(H,53,54) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017696
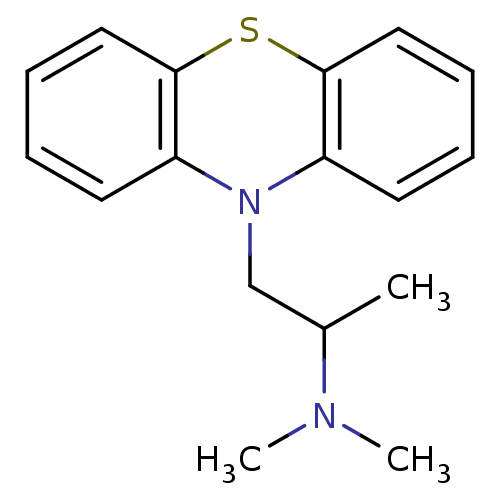
((2-dimethylamino-2-methyl)ethyl-N-dibenzoparathiaz...)Show InChI InChI=1S/C17H20N2S/c1-13(18(2)3)12-19-14-8-4-6-10-16(14)20-17-11-7-5-9-15(17)19/h4-11,13H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor (unknown origin) by radioligand binding assay |
J Med Chem 56: 8955-71 (2013)
Article DOI: 10.1021/jm400856t
BindingDB Entry DOI: 10.7270/Q28C9XPF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290728

(CHEMBL96441 | [3-{2-[4-(1H-Benzoimidazole-2-carbon...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3[nH]2)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C37H44N4O7/c1-44-29-11-10-26(22-30(29)45-2)37(15-19-41(23-37)36(43)25-20-31(46-3)34(48-5)32(21-25)47-4)14-18-40-16-12-24(13-17-40)33(42)35-38-27-8-6-7-9-28(27)39-35/h6-11,20-22,24H,12-19,23H2,1-5H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290720

(4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoyl)-...)Show SMILES COC(=O)c1ccc(Cn2c(nc3ccccc23)C(=O)C2CCN(CCC3(CCN(C3)C(=O)c3cc(OC)c(OC)c(OC)c3)c3ccccc3)CC2)cc1 Show InChI InChI=1S/C44H48N4O7/c1-52-37-26-33(27-38(53-2)40(37)54-3)42(50)47-25-21-44(29-47,34-10-6-5-7-11-34)20-24-46-22-18-31(19-23-46)39(49)41-45-35-12-8-9-13-36(35)48(41)28-30-14-16-32(17-15-30)43(51)55-4/h5-17,26-27,31H,18-25,28-29H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403978
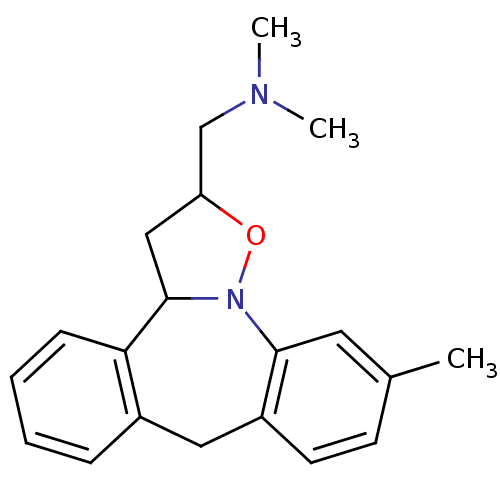
(CHEMBL85735)Show InChI InChI=1S/C20H24N2O/c1-14-8-9-16-11-15-6-4-5-7-18(15)20-12-17(13-21(2)3)23-22(20)19(16)10-14/h4-10,17,20H,11-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290726
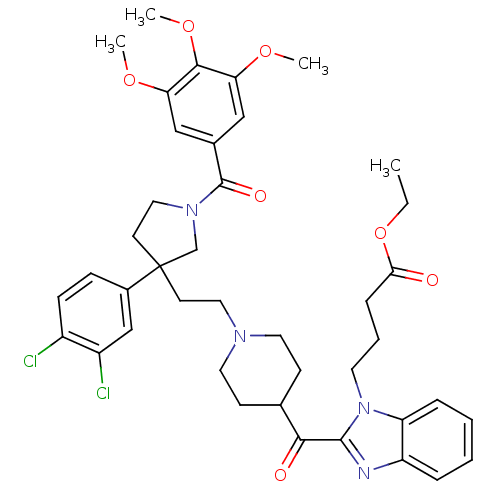
(4-[2-(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trime...)Show SMILES CCOC(=O)CCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C41H48Cl2N4O7/c1-5-54-36(48)11-8-18-47-33-10-7-6-9-32(33)44-39(47)37(49)27-14-19-45(20-15-27)21-16-41(29-12-13-30(42)31(43)25-29)17-22-46(26-41)40(50)28-23-34(51-2)38(53-4)35(24-28)52-3/h6-7,9-10,12-13,23-25,27H,5,8,11,14-22,26H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290727
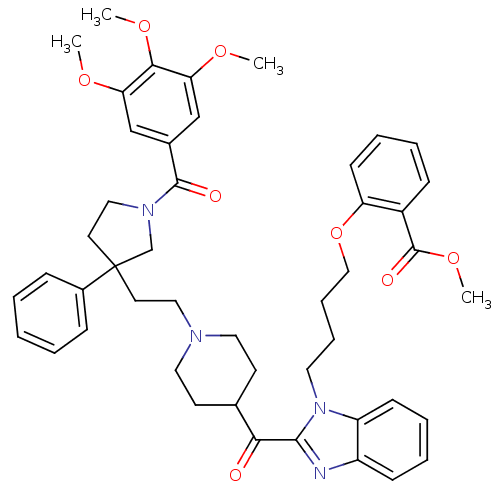
(2-{4-[2-(1-{2-[3-Phenyl-1-(3,4,5-trimethoxy-benzoy...)Show SMILES COC(=O)c1ccccc1OCCCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccccc2)CC1 Show InChI InChI=1S/C47H54N4O8/c1-55-40-30-34(31-41(56-2)43(40)57-3)45(53)50-28-23-47(32-50,35-14-6-5-7-15-35)22-27-49-25-20-33(21-26-49)42(52)44-48-37-17-9-10-18-38(37)51(44)24-12-13-29-59-39-19-11-8-16-36(39)46(54)58-4/h5-11,14-19,30-31,33H,12-13,20-29,32H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290725

(4-[2-(1-{2-[3-(3,4-Dimethoxy-phenyl)-1-(3,4,5-trim...)Show SMILES COC(=O)c1ccc(Cn2c(nc3ccccc23)C(=O)C2CCN(CCC3(CCN(C3)C(=O)c3cc(OC)c(OC)c(OC)c3)c3ccc(OC)c(OC)c3)CC2)cc1 Show InChI InChI=1S/C46H52N4O9/c1-54-37-16-15-34(27-38(37)55-2)46(20-24-49(29-46)44(52)33-25-39(56-3)42(58-5)40(26-33)57-4)19-23-48-21-17-31(18-22-48)41(51)43-47-35-9-7-8-10-36(35)50(43)28-30-11-13-32(14-12-30)45(53)59-6/h7-16,25-27,31H,17-24,28-29H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50403984
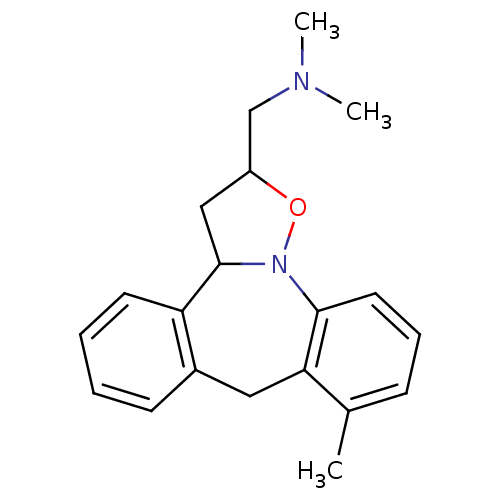
(CHEMBL82635)Show InChI InChI=1S/C20H24N2O/c1-14-7-6-10-19-18(14)11-15-8-4-5-9-17(15)20-12-16(13-21(2)3)23-22(19)20/h4-10,16,20H,11-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned Histamine H1 receptor expressed in CHO cells by [3H]pyrilamine displacement. |
Bioorg Med Chem Lett 12: 249-53 (2001)
Article DOI: 10.1016/S0960-894X(01)00722-3
BindingDB Entry DOI: 10.7270/Q2ZW1N3V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290729

(CHEMBL327888 | [3-(3,4-Dimethoxy-phenyl)-3-(2-{4-[...)Show SMILES CCOCCn1c(nc2ccccc12)C(=O)C1CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccc(OC)c(OC)c2)CC1 Show InChI InChI=1S/C41H52N4O8/c1-7-53-23-22-45-32-11-9-8-10-31(32)42-39(45)37(46)28-14-18-43(19-15-28)20-16-41(30-12-13-33(48-2)34(26-30)49-3)17-21-44(27-41)40(47)29-24-35(50-4)38(52-6)36(25-29)51-5/h8-13,24-26,28H,7,14-23,27H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529276

(CHEMBL4587694)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H29ClN2O2/c29-23-10-11-26-22(17-23)9-8-21-5-4-14-30-28(21)27(26)20-12-15-31(16-13-20)18-24(32)19-33-25-6-2-1-3-7-25/h1-7,10-11,14,17,24,32H,8-9,12-13,15-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290714

((3-(4-Methoxy-phenyl)-3-{2-[4-(1-pyridin-2-ylmethy...)Show SMILES COc1ccc(cc1)C1(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2Cc2ccccn2)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C42H47N5O6/c1-50-33-14-12-31(13-15-33)42(19-24-46(28-42)41(49)30-25-36(51-2)39(53-4)37(26-30)52-3)18-23-45-21-16-29(17-22-45)38(48)40-44-34-10-5-6-11-35(34)47(40)27-32-9-7-8-20-43-32/h5-15,20,25-26,29H,16-19,21-24,27-28H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290705

((3-{2-[4-(1H-Benzoimidazole-2-carbonyl)-piperidin-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3[nH]2)(C1)c1ccccc1 Show InChI InChI=1S/C35H40N4O5/c1-42-29-21-25(22-30(43-2)32(29)44-3)34(41)39-20-16-35(23-39,26-9-5-4-6-10-26)15-19-38-17-13-24(14-18-38)31(40)33-36-27-11-7-8-12-28(27)37-33/h4-12,21-22,24H,13-20,23H2,1-3H3,(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50290736
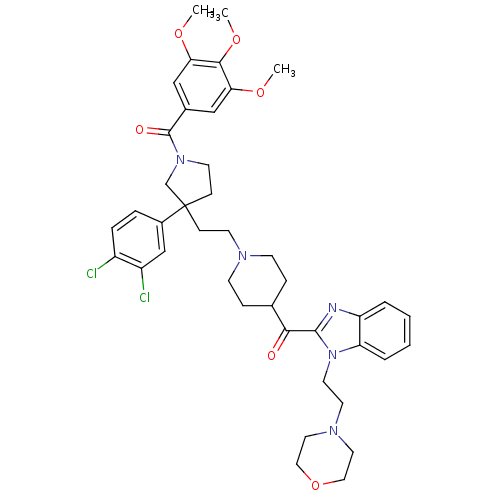
(CHEMBL97240 | [3-(3,4-Dichloro-phenyl)-3-(2-{4-[1-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)C(=O)c2nc3ccccc3n2CCN2CCOCC2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C41H49Cl2N5O6/c1-51-35-24-29(25-36(52-2)38(35)53-3)40(50)47-17-13-41(27-47,30-8-9-31(42)32(43)26-30)12-16-45-14-10-28(11-15-45)37(49)39-44-33-6-4-5-7-34(33)48(39)19-18-46-20-22-54-23-21-46/h4-9,24-26,28H,10-23,27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against histamine H1 receptor |
Bioorg Med Chem Lett 7: 2825-2830 (1997)
Article DOI: 10.1016/S0960-894X(97)10098-1
BindingDB Entry DOI: 10.7270/Q2SF2W50 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data