 Found 17 hits of ic50 for UniProtKB: P23204
Found 17 hits of ic50 for UniProtKB: P23204 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50030474
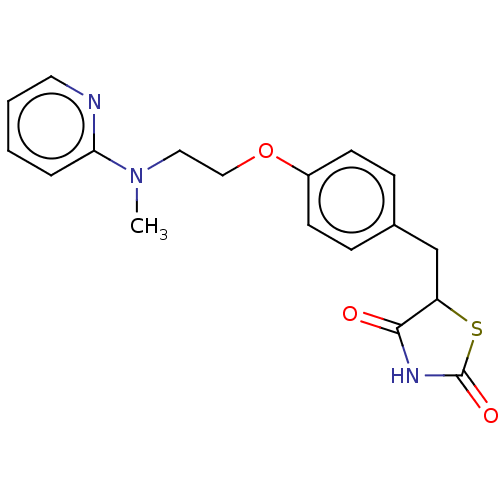
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50156523
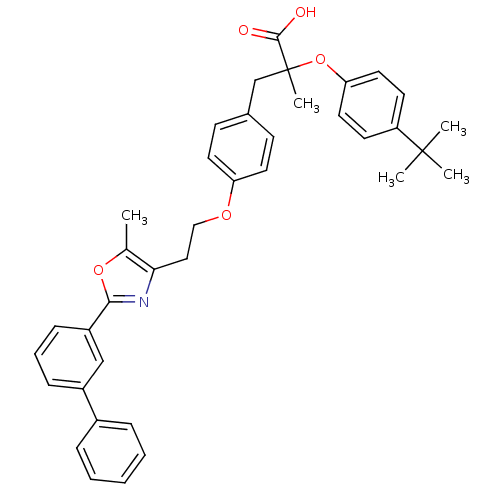
(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C38H39NO5/c1-26-34(39-35(43-26)30-13-9-12-29(24-30)28-10-7-6-8-11-28)22-23-42-32-18-14-27(15-19-32)25-38(5,36(40)41)44-33-20-16-31(17-21-33)37(2,3)4/h6-21,24H,22-23,25H2,1-5H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50145723
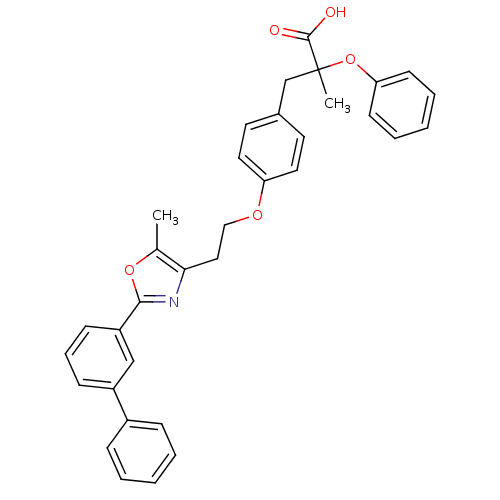
(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C34H31NO5/c1-24-31(35-32(39-24)28-13-9-12-27(22-28)26-10-5-3-6-11-26)20-21-38-29-18-16-25(17-19-29)23-34(2,33(36)37)40-30-14-7-4-8-15-30/h3-19,22H,20-21,23H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50156527

(2-(4-tert-Butyl-phenoxy)-2-methyl-3-{4-[2-(5-methy...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C32H35NO5/c1-22-28(33-29(37-22)24-9-7-6-8-10-24)19-20-36-26-15-11-23(12-16-26)21-32(5,30(34)35)38-27-17-13-25(14-18-27)31(2,3)4/h6-18H,19-21H2,1-5H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 515 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50156524
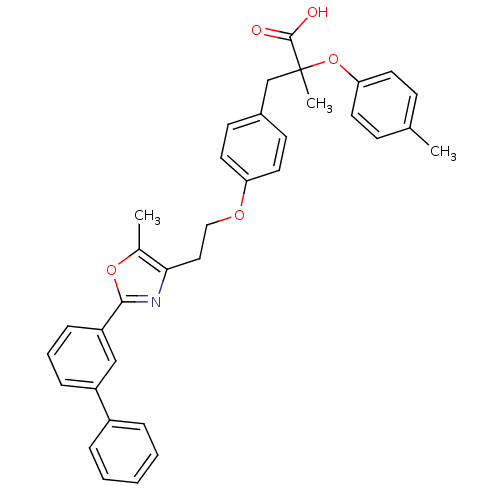
(3-{4-[2-(2-Biphenyl-3-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(C)cc2)C(O)=O)cc1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C35H33NO5/c1-24-12-16-31(17-13-24)41-35(3,34(37)38)23-26-14-18-30(19-15-26)39-21-20-32-25(2)40-33(36-32)29-11-7-10-28(22-29)27-8-5-4-6-9-27/h4-19,22H,20-21,23H2,1-3H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50200411
((S)-2-(5-(3-((2-(4-ethylphenyl)-5-methylpyrimidin-...)Show SMILES CCc1ccc(cc1)-c1ncc(C)c(n1)N(C)CCCOc1ccc2[C@H](CC(O)=O)CCc2c1 Show InChI InChI=1S/C28H33N3O3/c1-4-20-6-8-21(9-7-20)27-29-18-19(2)28(30-27)31(3)14-5-15-34-24-12-13-25-22(16-24)10-11-23(25)17-26(32)33/h6-9,12-13,16,18,23H,4-5,10-11,14-15,17H2,1-3H3,(H,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Activity at mouse PPARalpha expressed in CV1 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 1056-61 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.025
BindingDB Entry DOI: 10.7270/Q2CF9PR2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50156526
(3-{4-[2-(2-Biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C38H39NO5/c1-26-34(39-35(43-26)30-15-13-29(14-16-30)28-9-7-6-8-10-28)23-24-42-32-19-11-27(12-20-32)25-38(5,36(40)41)44-33-21-17-31(18-22-33)37(2,3)4/h6-22H,23-25H2,1-5H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50103521
(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50508127
(CHEMBL4449687)Show SMILES CCOc1ccc(cc1CC(O)=O)-c1ccc(CCCc2nn(-c3ccc(cc3)C(F)(F)F)c(=O)n2-c2ccccc2F)cc1 |(50.85,-41.06,;49.52,-41.85,;48.18,-41.09,;46.86,-41.88,;45.51,-41.12,;44.19,-41.9,;44.21,-43.43,;45.54,-44.2,;46.87,-43.42,;48.21,-44.18,;48.22,-45.72,;46.9,-46.5,;49.56,-46.47,;42.88,-44.22,;42.89,-45.76,;41.57,-46.54,;40.23,-45.77,;38.9,-46.55,;37.56,-45.8,;36.23,-46.58,;34.89,-45.82,;34.41,-44.35,;32.87,-44.36,;31.96,-43.12,;32.59,-41.71,;31.68,-40.47,;30.15,-40.63,;29.53,-42.05,;30.44,-43.29,;29.24,-39.39,;29.86,-37.98,;27.71,-39.56,;28.14,-38.29,;32.4,-45.83,;30.94,-46.31,;33.65,-46.72,;33.66,-48.26,;34.99,-49.03,;35,-50.58,;33.66,-51.35,;32.33,-50.58,;32.33,-49.03,;31,-48.26,;40.21,-44.24,;41.53,-43.46,)| Show InChI InChI=1S/C34H29F4N3O4/c1-2-45-30-19-14-24(20-25(30)21-32(42)43)23-12-10-22(11-13-23)6-5-9-31-39-41(27-17-15-26(16-18-27)34(36,37)38)33(44)40(31)29-8-4-3-7-28(29)35/h3-4,7-8,10-20H,2,5-6,9,21H2,1H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse PPARalpha |
Bioorg Med Chem Lett 29: 503-508 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.045
BindingDB Entry DOI: 10.7270/Q2XK8JVQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50156525
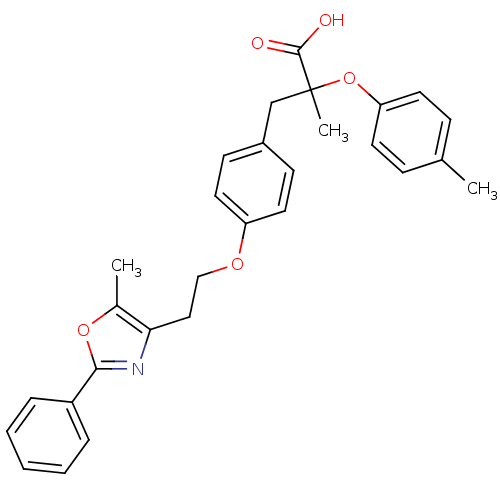
(2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(C)cc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C29H29NO5/c1-20-9-13-25(14-10-20)35-29(3,28(31)32)19-22-11-15-24(16-12-22)33-18-17-26-21(2)34-27(30-26)23-7-5-4-6-8-23/h4-16H,17-19H2,1-3H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50156522
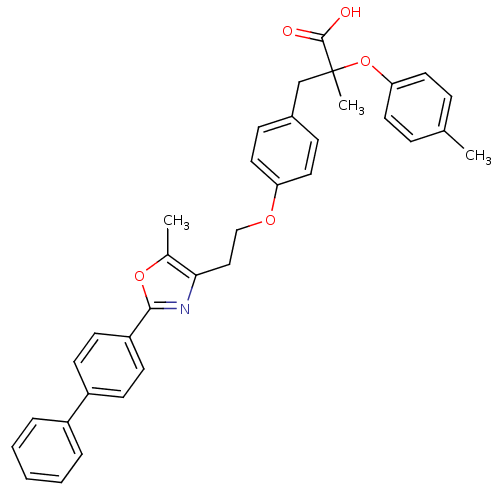
(3-{4-[2-(2-Biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccc(C)cc2)C(O)=O)cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C35H33NO5/c1-24-9-17-31(18-10-24)41-35(3,34(37)38)23-26-11-19-30(20-12-26)39-22-21-32-25(2)40-33(36-32)29-15-13-28(14-16-29)27-7-5-4-6-8-27/h4-20H,21-23H2,1-3H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50145722
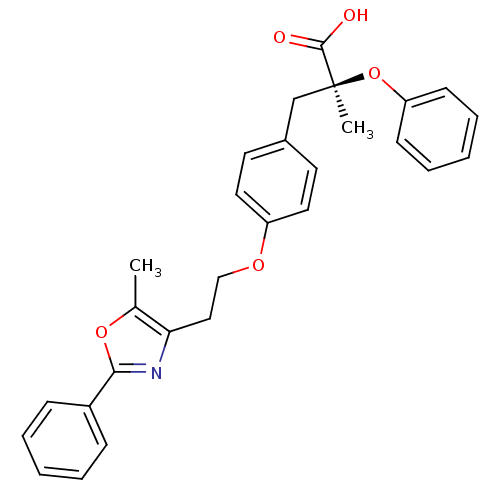
((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@](C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50200403
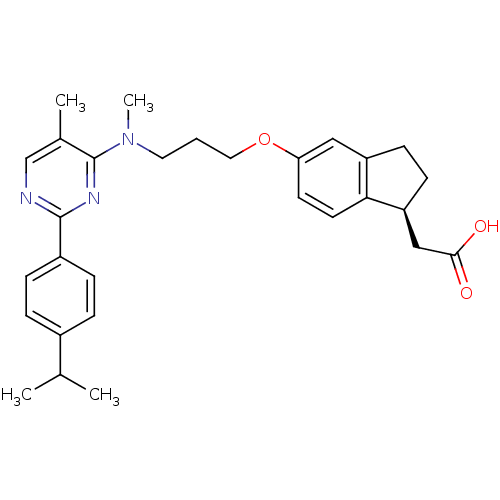
((S)-2-(5-(3-((2-(4-isopropylphenyl)-5-methylpyrimi...)Show SMILES CC(C)c1ccc(cc1)-c1ncc(C)c(n1)N(C)CCCOc1ccc2[C@H](CC(O)=O)CCc2c1 Show InChI InChI=1S/C29H35N3O3/c1-19(2)21-6-8-22(9-7-21)28-30-18-20(3)29(31-28)32(4)14-5-15-35-25-12-13-26-23(16-25)10-11-24(26)17-27(33)34/h6-9,12-13,16,18-19,24H,5,10-11,14-15,17H2,1-4H3,(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Activity at mouse PPARalpha expressed in CV1 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 1056-61 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.025
BindingDB Entry DOI: 10.7270/Q2CF9PR2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50145712
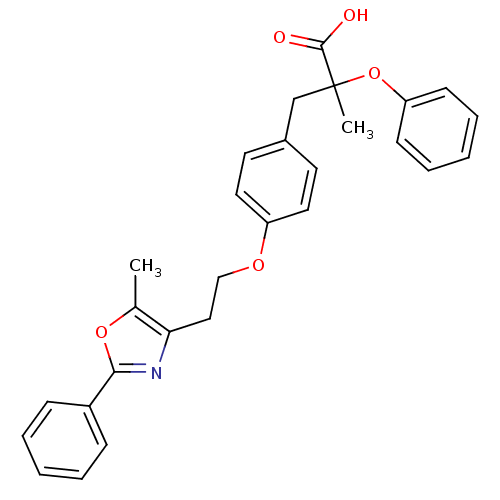
(2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50145715
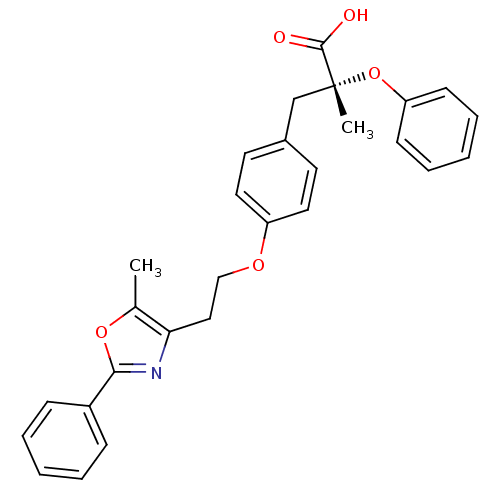
((R)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@](C)(Oc2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C28H27NO5/c1-20-25(29-26(33-20)22-9-5-3-6-10-22)17-18-32-23-15-13-21(14-16-23)19-28(2,27(30)31)34-24-11-7-4-8-12-24/h3-16H,17-19H2,1-2H3,(H,30,31)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50145714
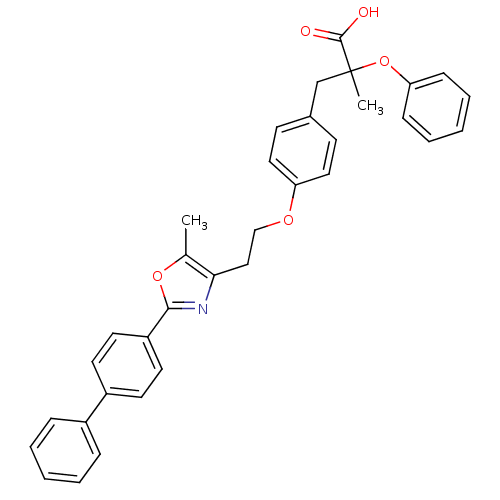
(3-{4-[2-(2-Biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...)Show SMILES Cc1oc(nc1CCOc1ccc(CC(C)(Oc2ccccc2)C(O)=O)cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C34H31NO5/c1-24-31(35-32(39-24)28-17-15-27(16-18-28)26-9-5-3-6-10-26)21-22-38-29-19-13-25(14-20-29)23-34(2,33(36)37)40-30-11-7-4-8-12-30/h3-20H,21-23H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of mouse Peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 14: 6113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.031
BindingDB Entry DOI: 10.7270/Q2QN67J1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50511113
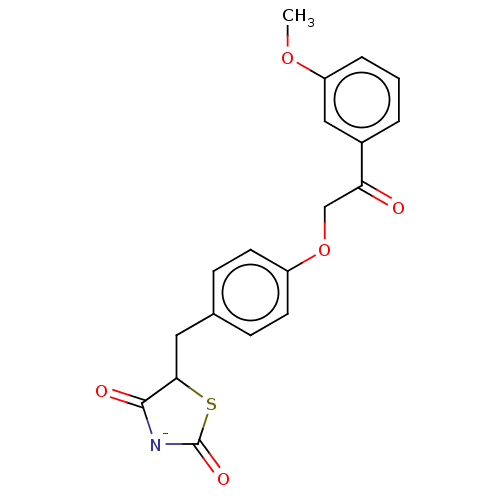
(CHEMBL4593380)Show SMILES [K;v0+].[#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#16]-[#6](=O)-[#7-]-[#6]-2=O)cc1 Show InChI InChI=1S/C19H17NO5S.K/c1-24-15-4-2-3-13(10-15)16(21)11-25-14-7-5-12(6-8-14)9-17-18(22)20-19(23)26-17;/h2-8,10,17H,9,11H2,1H3,(H,20,22,23);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data