

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50171881 ((E)-(R)-2-Amino-4-methyl-5-phosphono-pent-3-enoic ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]CPP binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50004927 (4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]CPP binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50132483 (CHEMBL108043 | Pentyl-(1-phenethyloxy-4,5-dihydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against N-methyl-D-aspartate glutamate receptor 2A in rat | Bioorg Med Chem Lett 13: 3155-9 (2003) BindingDB Entry DOI: 10.7270/Q2VT1RHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50010893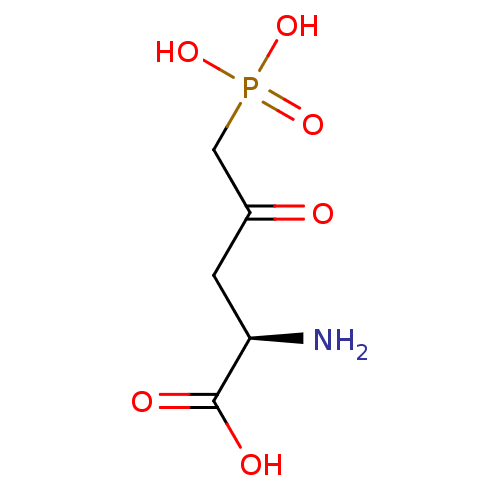 ((R)-2-Amino-4-oxo-5-phosphono-pentanoic acid | 2-A...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]CPP binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50399431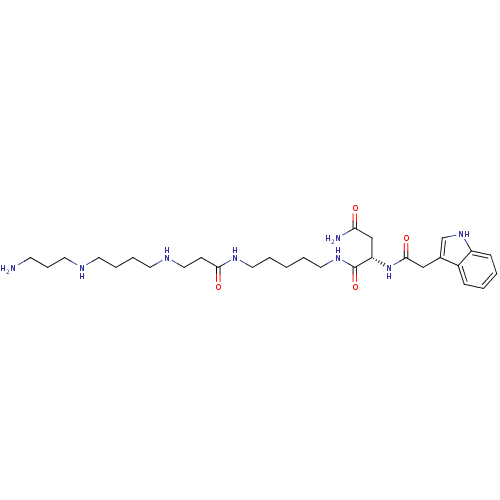 (CHEMBL2178919) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat glutamate N1/2A receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrop... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat glutamate N1/2A receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrop... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50132481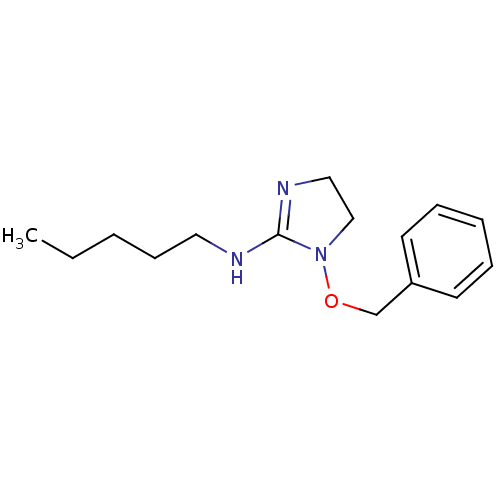 ((1-Benzyloxy-4,5-dihydro-1H-imidazol-2-yl)-pentyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against N-methyl-D-aspartate glutamate receptor 2A in rat | Bioorg Med Chem Lett 13: 3155-9 (2003) BindingDB Entry DOI: 10.7270/Q2VT1RHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50171881 ((E)-(R)-2-Amino-4-methyl-5-phosphono-pent-3-enoic ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]Glu binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50171882 ((E)-(R)-2-Amino-4-phosphonomethyl-hept-3-enoic aci...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]Glu binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50017229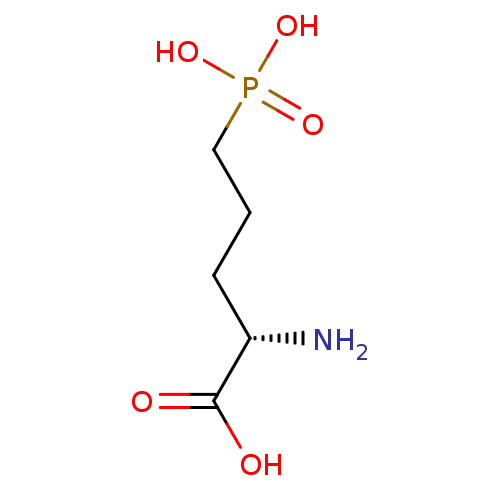 ((S)-2-Amino-5-phosphono-pentanoic acid | (S)-2-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]CPP binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50004927 (4-Phosphonomethyl-piperidine-2-carboxylic acid | 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]Glu binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50242450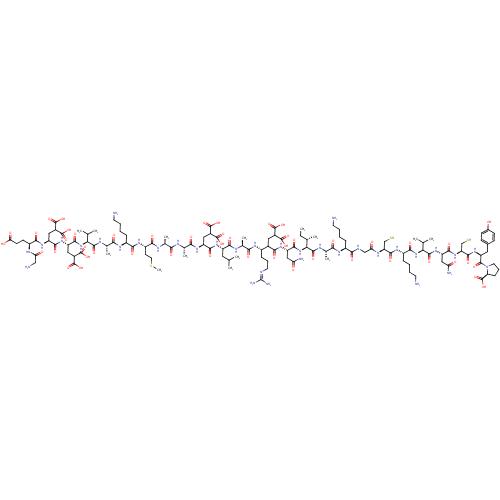 (CHEMBL524886 | conantokin-R) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of rat NMDA NR2A receptor expressed in xenopus oocytes coexpressing NMDA NR13b assessed as effect on glycine-induced current response at -... | J Biol Chem 282: 36905-13 (2007) Article DOI: 10.1074/jbc.M706611200 BindingDB Entry DOI: 10.7270/Q2NP246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50399430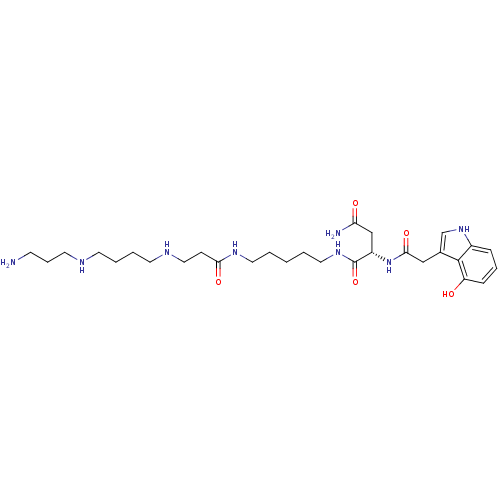 (CHEMBL2178920) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat glutamate N1/2A receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrop... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50017229 ((S)-2-Amino-5-phosphono-pentanoic acid | (S)-2-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of [3H]Glu binding to rat N-methyl-D-aspartic acid receptor 2A | J Med Chem 48: 5489-94 (2005) Article DOI: 10.1021/jm050174x BindingDB Entry DOI: 10.7270/Q2HD7WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50105843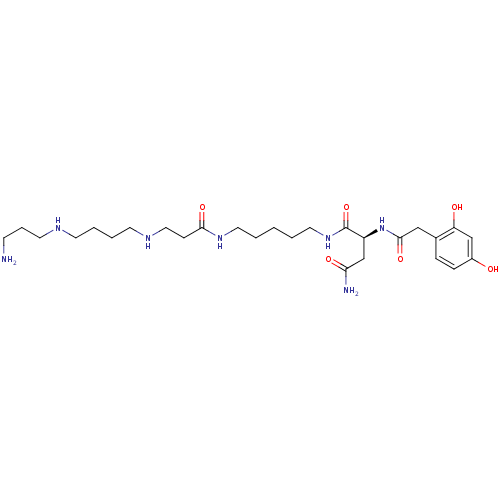 (2-Amino-N-{3-[4-(3-amino-propylamino)-butylamino]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat glutamate N1/2A receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrop... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440067 (CHEMBL2426072 | CHEMBL2426073) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50399433 (CHEMBL2178917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat glutamate N1/2A receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrop... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440072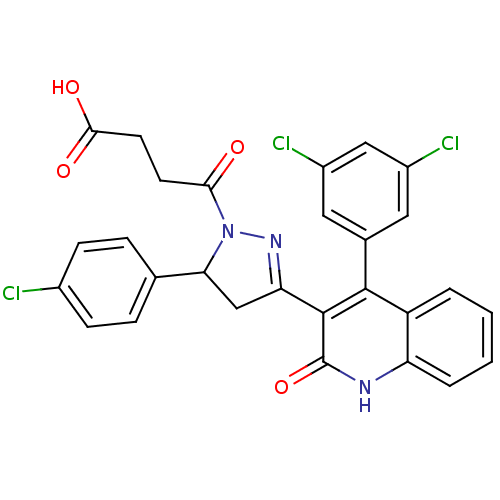 (CHEMBL2426067) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440075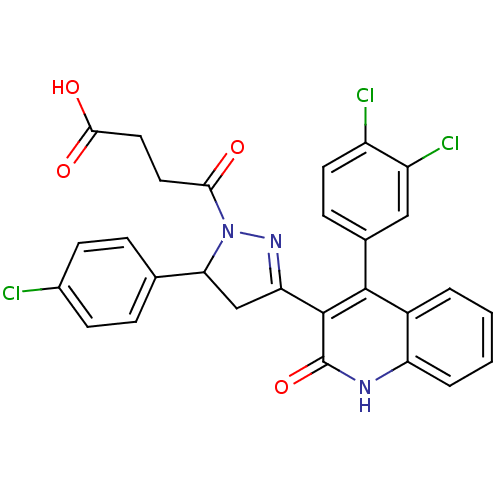 (CHEMBL2425979) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50242447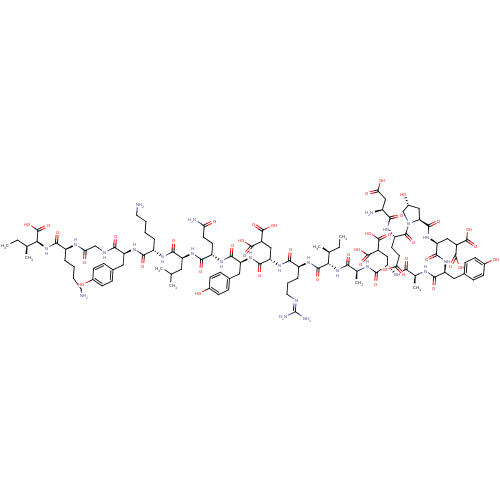 (CHEMBL525783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of rat NMDA NR2A receptor expressed in xenopus oocytes coexpressing NMDA NR13b assessed as effect on glycine-induced current response at -... | J Biol Chem 282: 36905-13 (2007) Article DOI: 10.1074/jbc.M706611200 BindingDB Entry DOI: 10.7270/Q2NP246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50242448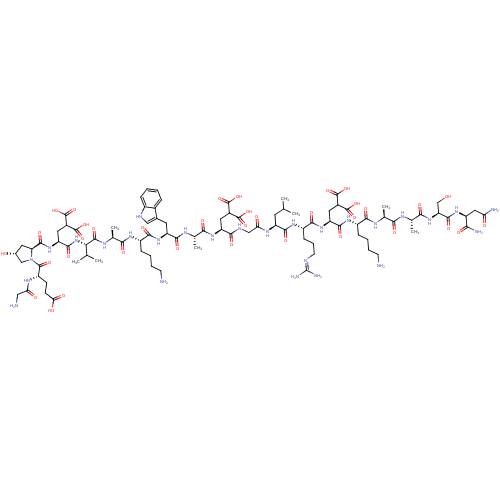 (CHEMBL526324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of rat NMDA NR2A receptor expressed in xenopus oocytes coexpressing NMDA NR13b assessed as effect on glycine-induced current response at -... | J Biol Chem 282: 36905-13 (2007) Article DOI: 10.1074/jbc.M706611200 BindingDB Entry DOI: 10.7270/Q2NP246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50242449 (CHEMBL525025 | conantokin-G) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of rat NMDA NR2A receptor expressed in xenopus oocytes coexpressing NMDA NR13b assessed as effect on glycine-induced current response at -... | J Biol Chem 282: 36905-13 (2007) Article DOI: 10.1074/jbc.M706611200 BindingDB Entry DOI: 10.7270/Q2NP246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50103133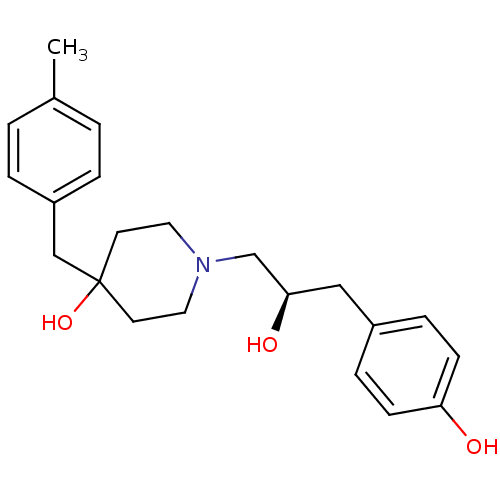 (1-[(R)-2-Hydroxy-3-(4-hydroxy-phenyl)-propyl]-4-(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Evaluated for in vitro inhibition of recombinant rat NR1C/2A receptor expressed in xenopus oocytes | Bioorg Med Chem Lett 11: 2173-6 (2001) BindingDB Entry DOI: 10.7270/Q2GF0SS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50399432 (CHEMBL2178918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat glutamate N1/2A receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrop... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50242446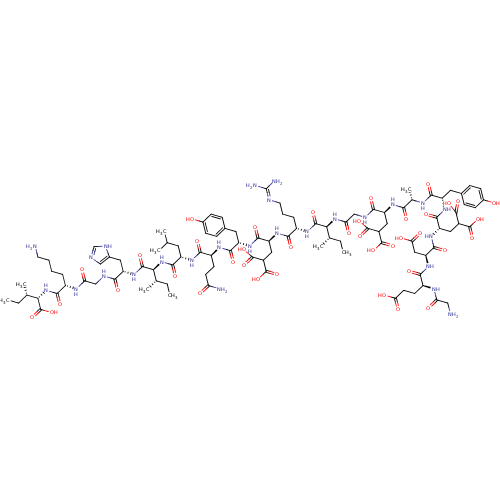 (CHEMBL524338) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of rat NMDA NR2A receptor expressed in xenopus oocytes coexpressing NMDA NR13b assessed as effect on glycine-induced current response at -... | J Biol Chem 282: 36905-13 (2007) Article DOI: 10.1074/jbc.M706611200 BindingDB Entry DOI: 10.7270/Q2NP246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440084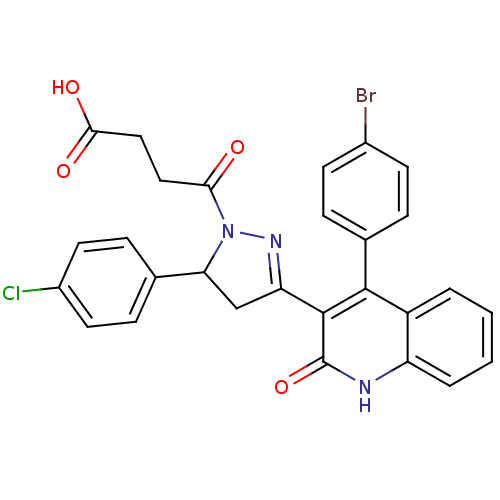 (CHEMBL2426093) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440065 (CHEMBL2426075) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440077 (CHEMBL2425977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440067 (CHEMBL2426072 | CHEMBL2426073) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440060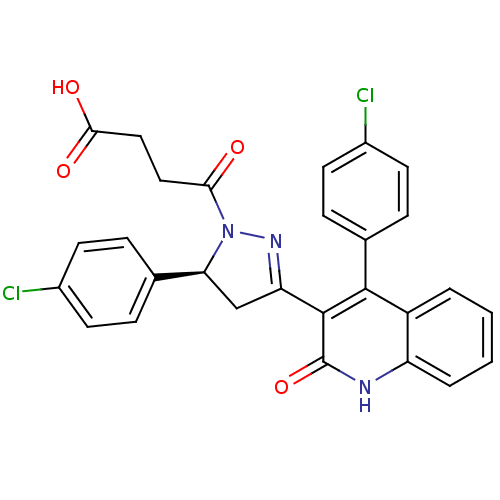 (CHEMBL2426081) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440085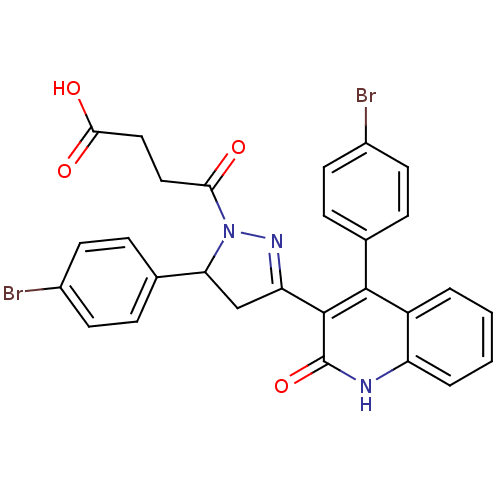 (CHEMBL2426092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440081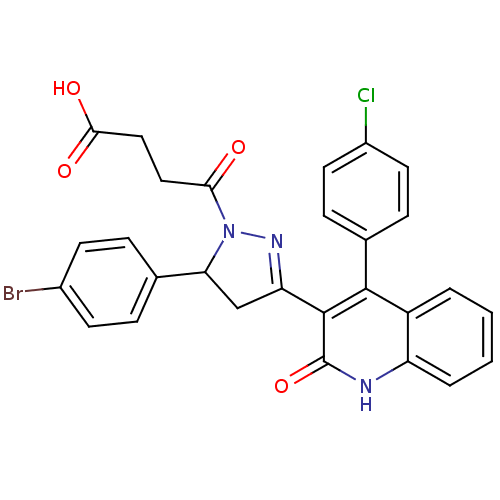 (CHEMBL2426096) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440076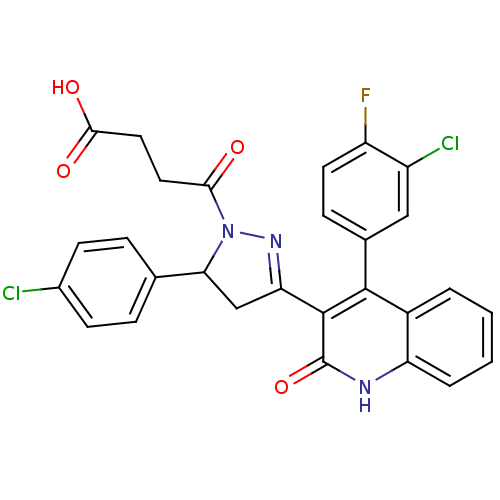 (CHEMBL2425978) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440051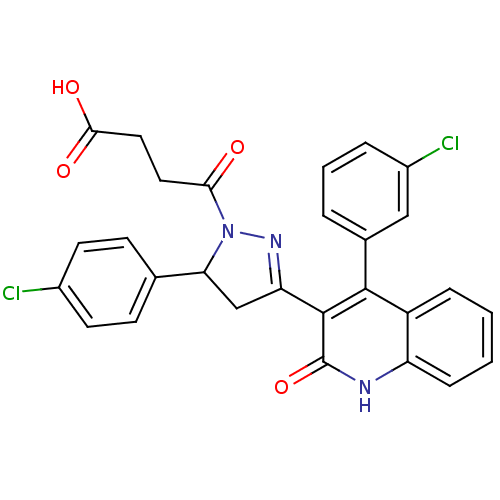 (CHEMBL2425972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440073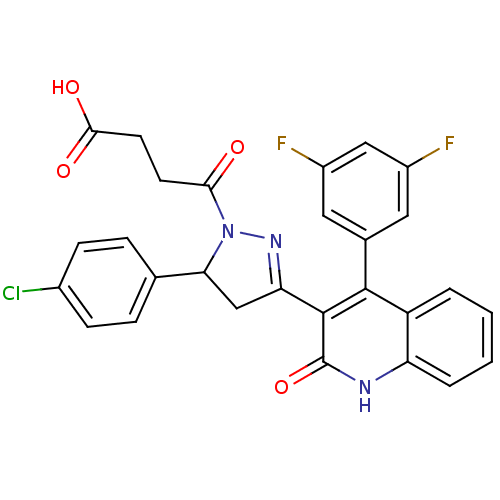 (CHEMBL2426066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440058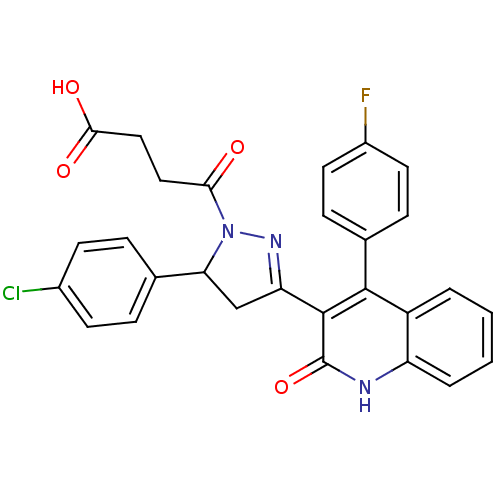 (CHEMBL2426100) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440074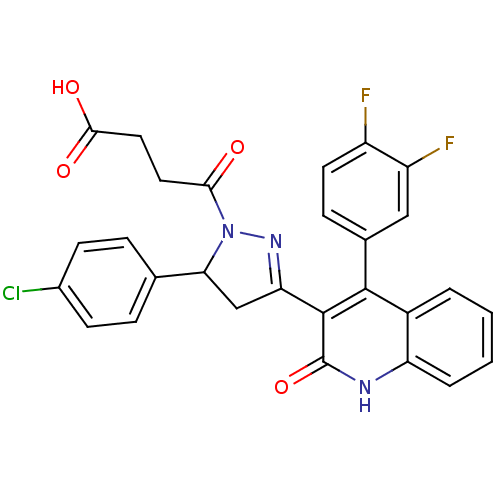 (CHEMBL2426065) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440057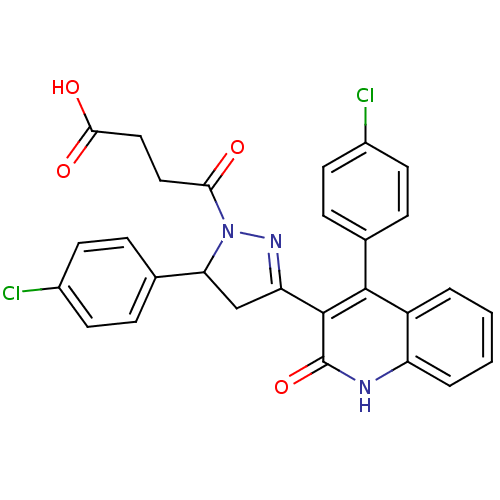 (CHEMBL2426097) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440050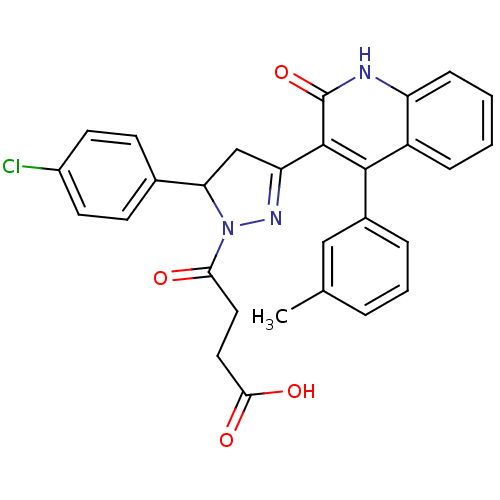 (CHEMBL2425973) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440047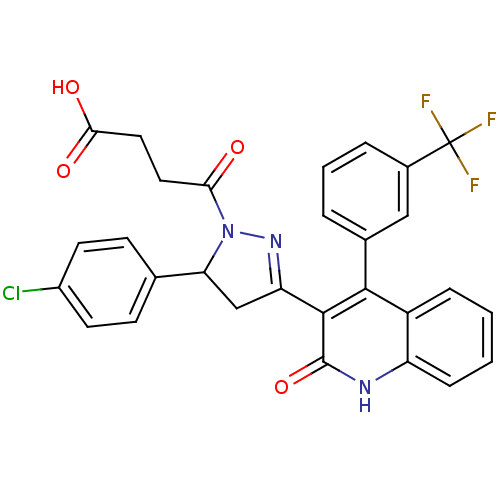 (CHEMBL2425976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440053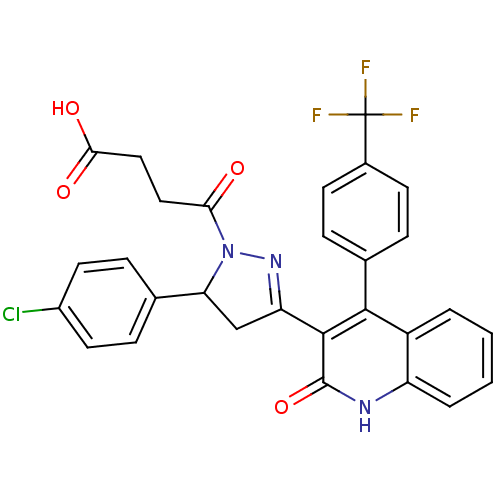 (CHEMBL2426104) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440078 (CHEMBL2426101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440062 (CHEMBL2426078) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440083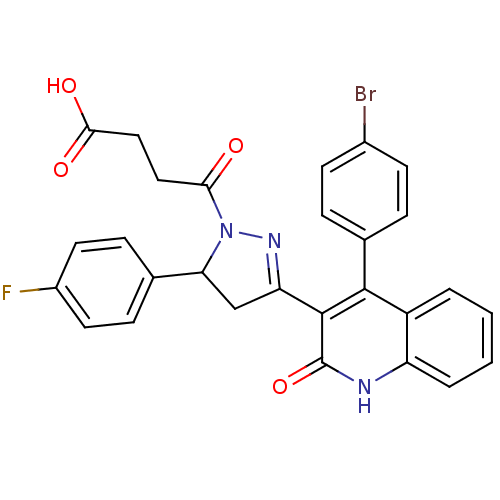 (CHEMBL2426094) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440059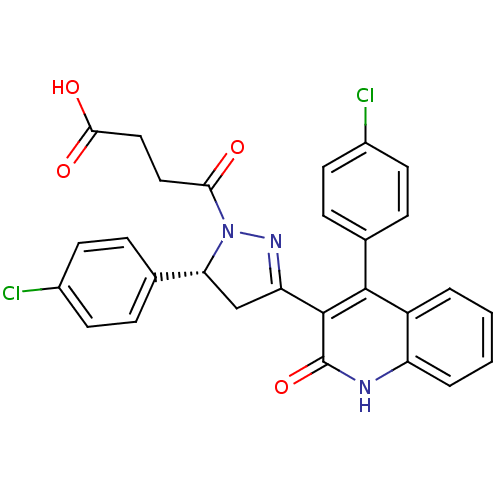 (CHEMBL2426082) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440052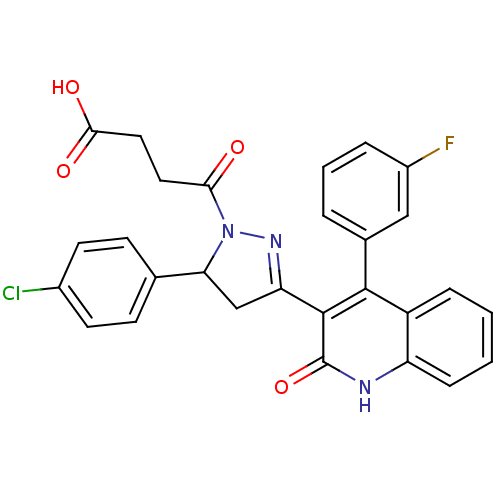 (CHEMBL2426105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440080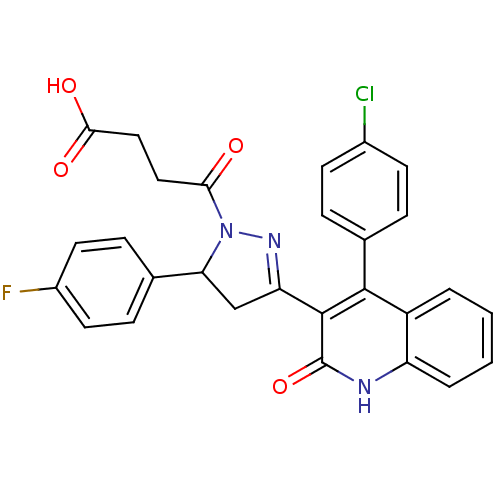 (CHEMBL2426098) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440079 (CHEMBL2426099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440061 (CHEMBL2424674) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2A (Rattus norvegicus (Rat)) | BDBM50440063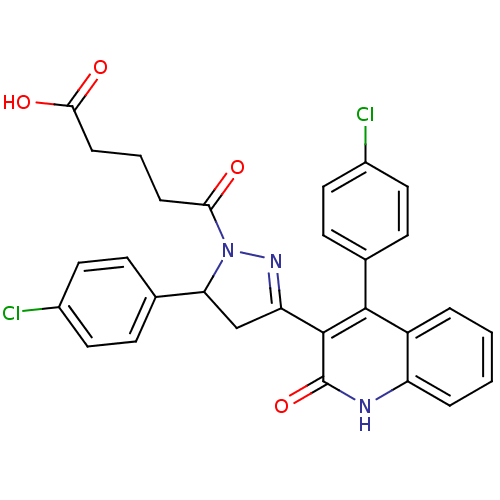 (CHEMBL2426077) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Negative allosteric modulation of recombinant rat GluN2A receptor expressed in Xenopus laevis oocytes by two-electrode voltage clamp method | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |