 Found 76 hits of ki for UniProtKB: P00813
Found 76 hits of ki for UniProtKB: P00813 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

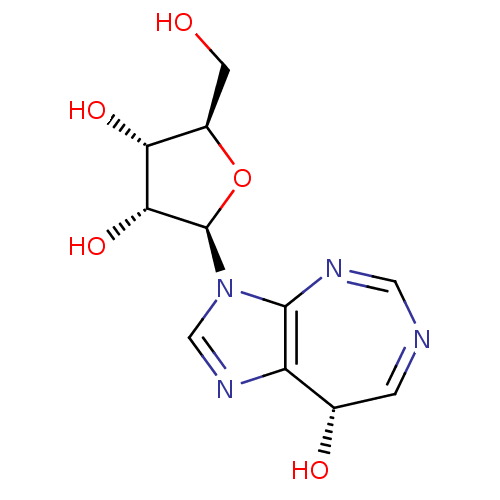
((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
Bioorg Med Chem Lett 22: 7214-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.053
BindingDB Entry DOI: 10.7270/Q2MC916H |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032
(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
Bioorg Med Chem Lett 22: 7214-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.053
BindingDB Entry DOI: 10.7270/Q2MC916H |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase. |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50407749

(CHEMBL2112110)Show SMILES OC[C@@H]1O[C@@H](C[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |c:17| Show InChI InChI=1S/C12H17N3O4/c16-5-9-8(18)4-10(19-9)15-6-14-11-7(17)2-1-3-13-12(11)15/h3,6-10,16-18H,1-2,4-5H2/t7-,8-,9+,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50378885
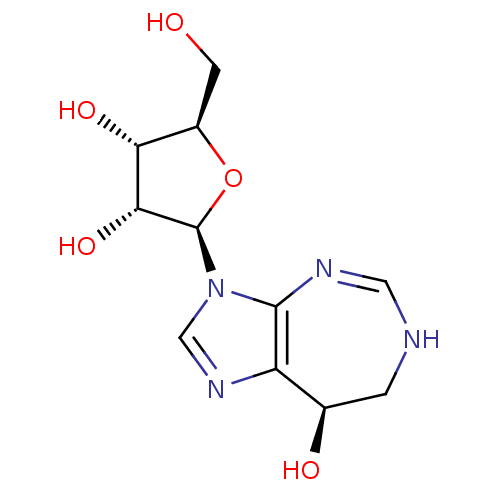
(CHEMBL1651378)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@@H](O)C=NC=Nc12 |r,c:15,17| Show InChI InChI=1S/C11H14N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h2,4-9,16-18H,1,3H2/t6-,7-,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
J Med Chem 54: 107-21 (2011)
Article DOI: 10.1021/jm101286g
BindingDB Entry DOI: 10.7270/Q2K64K1Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50378886
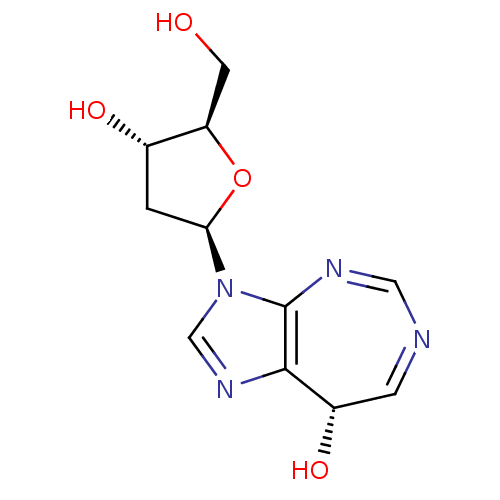
(CHEMBL1651377)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@@H](O)C=NC=Nc12 |r,c:16,18| Show InChI InChI=1S/C11H14N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h1,3-6,8-9,11,16-19H,2H2/t5-,6+,8+,9+,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
J Med Chem 54: 107-21 (2011)
Article DOI: 10.1021/jm101286g
BindingDB Entry DOI: 10.7270/Q2K64K1Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50369126
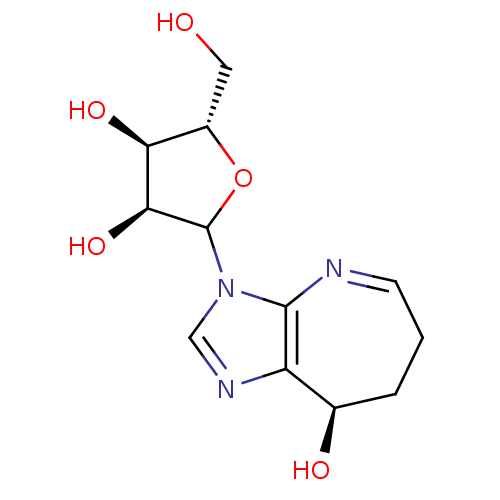
(CONFORMYCIN)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |r,c:18| Show InChI InChI=1S/C12H17N3O5/c16-4-7-9(18)10(19)12(20-7)15-5-14-8-6(17)2-1-3-13-11(8)15/h3,5-7,9-10,12,16-19H,1-2,4H2/t6-,7+,9+,10+,12?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0330 | -14.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
Bioorg Med Chem Lett 13: 1115-8 (2003)
Article DOI: 10.1016/S0960-894X(03)00026-X
BindingDB Entry DOI: 10.7270/Q29Z936D |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50370598
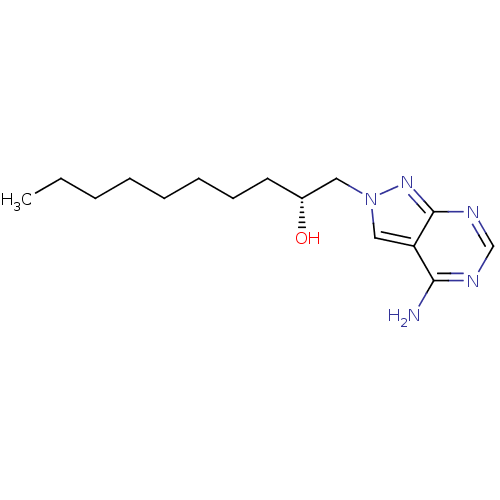
(CHEMBL1651379)Show InChI InChI=1S/C15H25N5O/c1-2-3-4-5-6-7-8-12(21)9-20-10-13-14(16)17-11-18-15(13)19-20/h10-12,21H,2-9H2,1H3,(H2,16,17,18,19)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
J Med Chem 54: 107-21 (2011)
Article DOI: 10.1021/jm101286g
BindingDB Entry DOI: 10.7270/Q2K64K1Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrate |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM28393
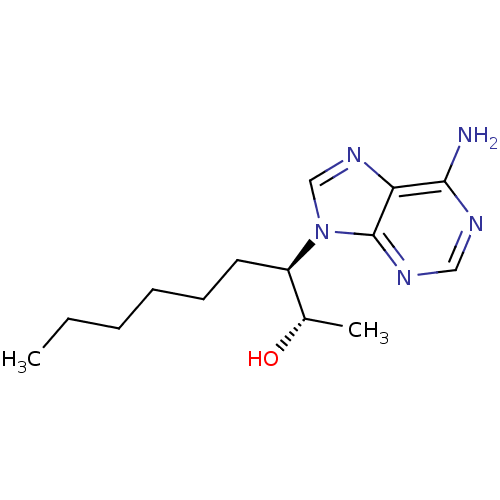
((+)-EHNA | (2S,3R)-3-(6-amino-9H-purin-9-yl)nonan-...)Show InChI InChI=1S/C14H23N5O/c1-3-4-5-6-7-11(10(2)20)19-9-18-12-13(15)16-8-17-14(12)19/h8-11,20H,3-7H2,1-2H3,(H2,15,16,17)/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM28393

((+)-EHNA | (2S,3R)-3-(6-amino-9H-purin-9-yl)nonan-...)Show InChI InChI=1S/C14H23N5O/c1-3-4-5-6-7-11(10(2)20)19-9-18-12-13(15)16-8-17-14(12)19/h8-11,20H,3-7H2,1-2H3,(H2,15,16,17)/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50034908

((2R,3S)-3-((R)-6-Amino-purin-9-yl)-nonan-2-ol | (2...)Show SMILES CCCCCC[C@@H]([C@@H](C)O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H23N5O/c1-3-4-5-6-7-11(10(2)20)19-9-18-12-13(15)16-8-17-14(12)19/h8-11,20H,3-7H2,1-2H3,(H2,15,16,17)/t10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Inhibition constant (Ki) against Human erythrocyte adenosine deaminase |
J Med Chem 31: 390-3 (1988)
BindingDB Entry DOI: 10.7270/Q2X067M7 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22947
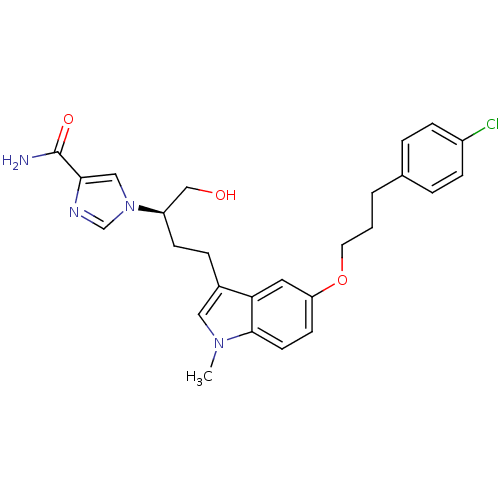
(1-[(2R)-4-{5-[3-(4-chlorophenyl)propoxy]-1-methyl-...)Show SMILES Cn1cc(CC[C@H](CO)n2cnc(c2)C(N)=O)c2cc(OCCCc3ccc(Cl)cc3)ccc12 |r| Show InChI InChI=1S/C26H29ClN4O3/c1-30-14-19(6-9-21(16-32)31-15-24(26(28)33)29-17-31)23-13-22(10-11-25(23)30)34-12-2-3-18-4-7-20(27)8-5-18/h4-5,7-8,10-11,13-15,17,21,32H,2-3,6,9,12,16H2,1H3,(H2,28,33)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | -11.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50011575
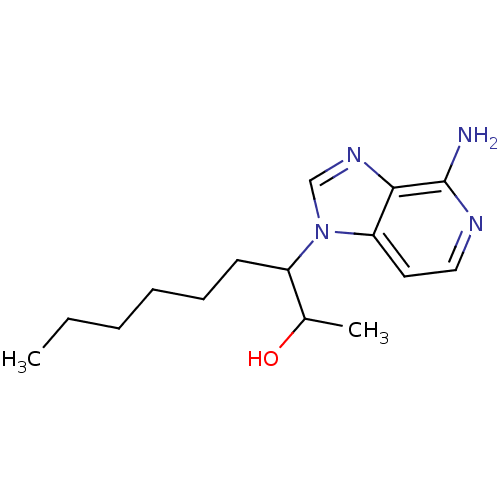
(3-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-nonan-2-ol ...)Show InChI InChI=1S/C15H24N4O/c1-3-4-5-6-7-12(11(2)20)19-10-18-14-13(19)8-9-17-15(14)16/h8-12,20H,3-7H2,1-2H3,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Inhibition constant (Ki) against Human erythrocyte adenosine deaminase |
J Med Chem 31: 390-3 (1988)
BindingDB Entry DOI: 10.7270/Q2X067M7 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22937
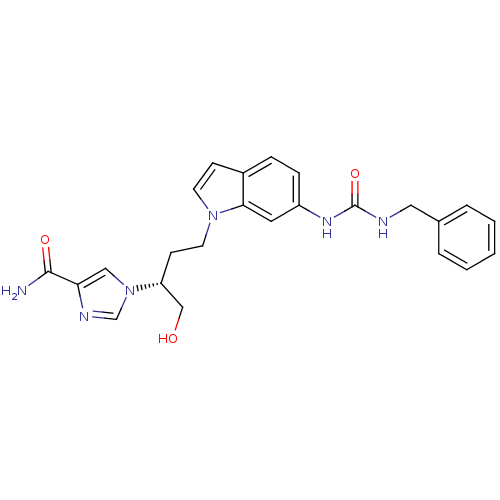
(1-[(2R)-4-{6-[(benzylcarbamoyl)amino]-1H-indol-1-y...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(NC(=O)NCc3ccccc3)cc12 |r| Show InChI InChI=1S/C24H26N6O3/c25-23(32)21-14-30(16-27-21)20(15-31)9-11-29-10-8-18-6-7-19(12-22(18)29)28-24(33)26-13-17-4-2-1-3-5-17/h1-8,10,12,14,16,20,31H,9,11,13,15H2,(H2,25,32)(H2,26,28,33)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.5 | -11.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22920

(1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...)Show SMILES Cn1c(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)nc2ccccc12 |r| Show InChI InChI=1S/C27H29N7O3/c1-32-23-5-3-2-4-21(23)31-25(32)8-9-26(36)30-19-7-6-18-10-12-33(24(18)14-19)13-11-20(16-35)34-15-22(27(28)37)29-17-34/h2-7,10,12,14-15,17,20,35H,8-9,11,13,16H2,1H3,(H2,28,37)(H,30,36)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | -10.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Am Chem Soc 126: 34-5 (2004)
Article DOI: 10.1021/ja038606l
BindingDB Entry DOI: 10.7270/Q2FQ9TWW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22920

(1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...)Show SMILES Cn1c(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)nc2ccccc12 |r| Show InChI InChI=1S/C27H29N7O3/c1-32-23-5-3-2-4-21(23)31-25(32)8-9-26(36)30-19-7-6-18-10-12-33(24(18)14-19)13-11-20(16-35)34-15-22(27(28)37)29-17-34/h2-7,10,12,14-15,17,20,35H,8-9,11,13,16H2,1H3,(H2,28,37)(H,30,36)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | -10.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22920

(1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...)Show SMILES Cn1c(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)nc2ccccc12 |r| Show InChI InChI=1S/C27H29N7O3/c1-32-23-5-3-2-4-21(23)31-25(32)8-9-26(36)30-19-7-6-18-10-12-33(24(18)14-19)13-11-20(16-35)34-15-22(27(28)37)29-17-34/h2-7,10,12,14-15,17,20,35H,8-9,11,13,16H2,1H3,(H2,28,37)(H,30,36)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | -10.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22920

(1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...)Show SMILES Cn1c(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)nc2ccccc12 |r| Show InChI InChI=1S/C27H29N7O3/c1-32-23-5-3-2-4-21(23)31-25(32)8-9-26(36)30-19-7-6-18-10-12-33(24(18)14-19)13-11-20(16-35)34-15-22(27(28)37)29-17-34/h2-7,10,12,14-15,17,20,35H,8-9,11,13,16H2,1H3,(H2,28,37)(H,30,36)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine deaminase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22950

(1-[(3R,4S)-4-hydroxy-1-(naphthalen-2-yloxy)pentan-...)Show SMILES C[C@H](O)[C@@H](CCOc1ccc2ccccc2c1)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C19H21N3O3/c1-13(23)18(22-11-17(19(20)24)21-12-22)8-9-25-16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10-13,18,23H,8-9H2,1H3,(H2,20,24)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 9.80 | -10.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50519494

(CHEMBL1234234)Show SMILES CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:19| Show InChI InChI=1S/C12H18N4O4S/c1-21-3-7-9(18)10(19)12(20-7)16-5-15-8-6(17)2-13-4-14-11(8)16/h4-7,9-10,12,17-19H,2-3H2,1H3,(H,13,14)/t6-,7-,9-,10-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrate |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22931
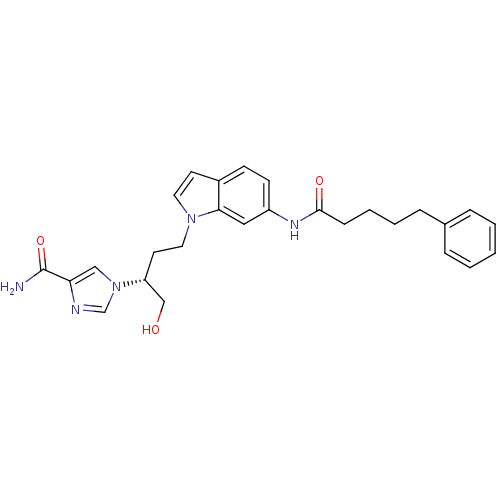
(1-[(2R)-1-hydroxy-4-[6-(5-phenylpentanamido)-1H-in...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(NC(=O)CCCCc3ccccc3)cc12 |r| Show InChI InChI=1S/C27H31N5O3/c28-27(35)24-17-32(19-29-24)23(18-33)13-15-31-14-12-21-10-11-22(16-25(21)31)30-26(34)9-5-4-8-20-6-2-1-3-7-20/h1-3,6-7,10-12,14,16-17,19,23,33H,4-5,8-9,13,15,18H2,(H2,28,35)(H,30,34)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -10.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22948
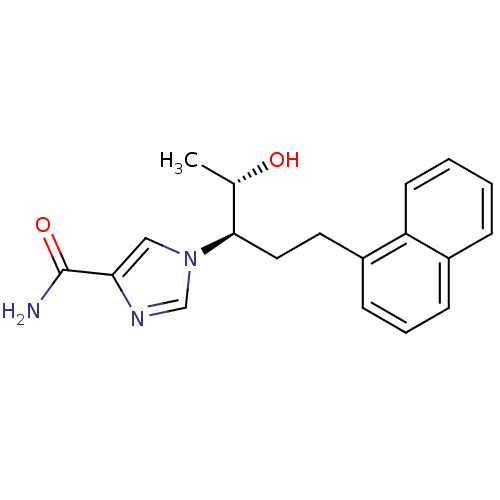
(1-[(1R,2S)-2-hydroxy-1-(2-naphthalen-1-ylethyl)pro...)Show SMILES C[C@H](O)[C@@H](CCc1cccc2ccccc12)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C19H21N3O2/c1-13(23)18(22-11-17(19(20)24)21-12-22)10-9-15-7-4-6-14-5-2-3-8-16(14)15/h2-8,11-13,18,23H,9-10H2,1H3,(H2,20,24)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -10.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22939
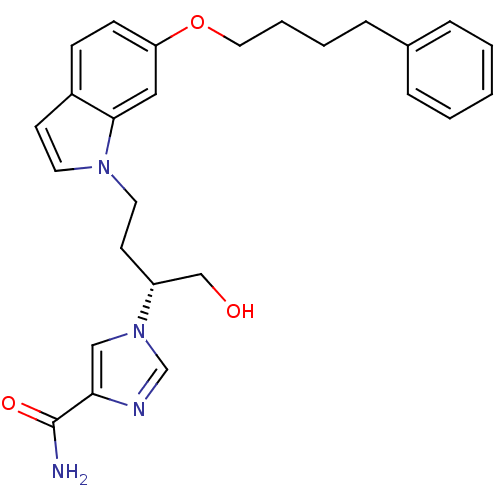
(1-[(2R)-1-hydroxy-4-[6-(4-phenylbutoxy)-1H-indol-1...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(OCCCCc3ccccc3)cc12 |r| Show InChI InChI=1S/C26H30N4O3/c27-26(32)24-17-30(19-28-24)22(18-31)12-14-29-13-11-21-9-10-23(16-25(21)29)33-15-5-4-8-20-6-2-1-3-7-20/h1-3,6-7,9-11,13,16-17,19,22,31H,4-5,8,12,14-15,18H2,(H2,27,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | -10.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22942

(1-[(2R)-4-{6-[3-(4-chlorophenyl)propoxy]-1H-indol-...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(OCCCc3ccc(Cl)cc3)cc12 |r| Show InChI InChI=1S/C25H27ClN4O3/c26-20-6-3-18(4-7-20)2-1-13-33-22-8-5-19-9-11-29(24(19)14-22)12-10-21(16-31)30-15-23(25(27)32)28-17-30/h3-9,11,14-15,17,21,31H,1-2,10,12-13,16H2,(H2,27,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | -10.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22949
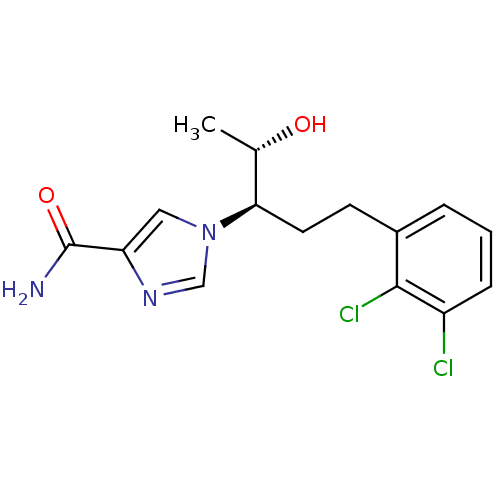
(1-[(3R,4S)-1-(2,3-dichlorophenyl)-4-hydroxypentan-...)Show SMILES C[C@H](O)[C@@H](CCc1cccc(Cl)c1Cl)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C15H17Cl2N3O2/c1-9(21)13(20-7-12(15(18)22)19-8-20)6-5-10-3-2-4-11(16)14(10)17/h2-4,7-9,13,21H,5-6H2,1H3,(H2,18,22)/t9-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 13 | -10.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
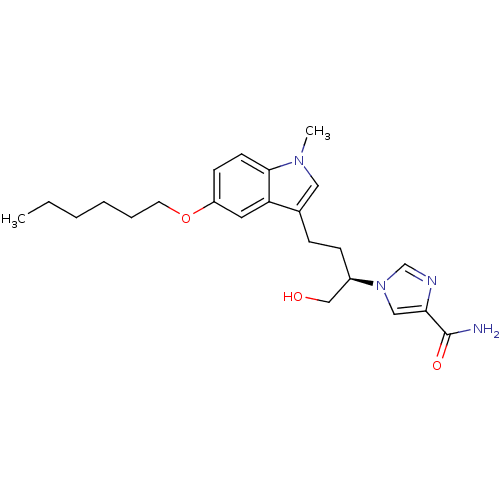
(Homo sapiens (Human)) | BDBM22946
(1-[(2R)-4-[5-(hexyloxy)-1-methyl-1H-indol-3-yl]-1-...)Show SMILES CCCCCCOc1ccc2n(C)cc(CC[C@H](CO)n3cnc(c3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C23H32N4O3/c1-3-4-5-6-11-30-19-9-10-22-20(12-19)17(13-26(22)2)7-8-18(15-28)27-14-21(23(24)29)25-16-27/h9-10,12-14,16,18,28H,3-8,11,15H2,1-2H3,(H2,24,29)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | -10.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrate |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22930

(1-[(2R)-1-hydroxy-4-[6-(4-phenylbutanamido)-1H-ind...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(NC(=O)CCCc3ccccc3)cc12 |r| Show InChI InChI=1S/C26H29N5O3/c27-26(34)23-16-31(18-28-23)22(17-32)12-14-30-13-11-20-9-10-21(15-24(20)30)29-25(33)8-4-7-19-5-2-1-3-6-19/h1-3,5-6,9-11,13,15-16,18,22,32H,4,7-8,12,14,17H2,(H2,27,34)(H,29,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | -10.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22936
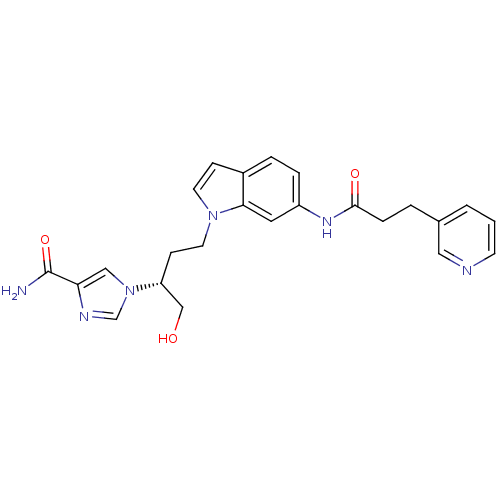
(1-[(2R)-1-hydroxy-4-{6-[3-(pyridin-3-yl)propanamid...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(NC(=O)CCc3cccnc3)cc12 |r| Show InChI InChI=1S/C24H26N6O3/c25-24(33)21-14-30(16-27-21)20(15-31)8-11-29-10-7-18-4-5-19(12-22(18)29)28-23(32)6-3-17-2-1-9-26-13-17/h1-2,4-5,7,9-10,12-14,16,20,31H,3,6,8,11,15H2,(H2,25,33)(H,28,32)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | -10.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22938

(1-[(2R)-1-hydroxy-4-[6-(3-phenylpropoxy)-1H-indol-...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(OCCCc3ccccc3)cc12 |r| Show InChI InChI=1S/C25H28N4O3/c26-25(31)23-16-29(18-27-23)21(17-30)11-13-28-12-10-20-8-9-22(15-24(20)28)32-14-4-7-19-5-2-1-3-6-19/h1-3,5-6,8-10,12,15-16,18,21,30H,4,7,11,13-14,17H2,(H2,26,31)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | -10.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22928

(1-[(2R)-4-(6-hexanamido-1H-indol-1-yl)-1-hydroxybu...)Show SMILES CCCCCC(=O)Nc1ccc2ccn(CC[C@H](CO)n3cnc(c3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C22H29N5O3/c1-2-3-4-5-21(29)25-17-7-6-16-8-10-26(20(16)12-17)11-9-18(14-28)27-13-19(22(23)30)24-15-27/h6-8,10,12-13,15,18,28H,2-5,9,11,14H2,1H3,(H2,23,30)(H,25,29)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
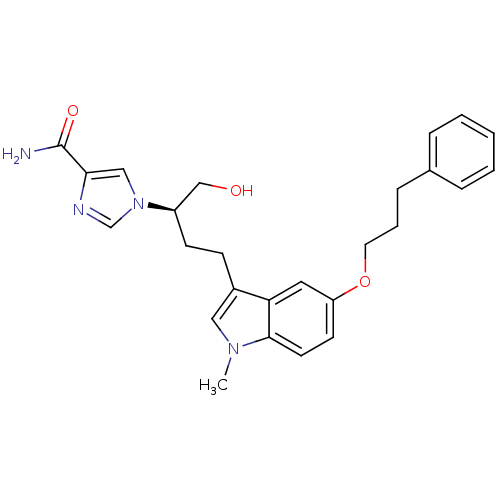
(Homo sapiens (Human)) | BDBM22945
(1-[(2R)-1-hydroxy-4-[1-methyl-5-(3-phenylpropoxy)-...)Show SMILES Cn1cc(CC[C@H](CO)n2cnc(c2)C(N)=O)c2cc(OCCCc3ccccc3)ccc12 |r| Show InChI InChI=1S/C26H30N4O3/c1-29-15-20(9-10-21(17-31)30-16-24(26(27)32)28-18-30)23-14-22(11-12-25(23)29)33-13-5-8-19-6-3-2-4-7-19/h2-4,6-7,11-12,14-16,18,21,31H,5,8-10,13,17H2,1H3,(H2,27,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | -10.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22929

(1-[(2R)-1-hydroxy-4-[6-(3-phenylpropanamido)-1H-in...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(NC(=O)CCc3ccccc3)cc12 |r| Show InChI InChI=1S/C25H27N5O3/c26-25(33)22-15-30(17-27-22)21(16-31)11-13-29-12-10-19-7-8-20(14-23(19)29)28-24(32)9-6-18-4-2-1-3-5-18/h1-5,7-8,10,12,14-15,17,21,31H,6,9,11,13,16H2,(H2,26,33)(H,28,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 30 | -10.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22935
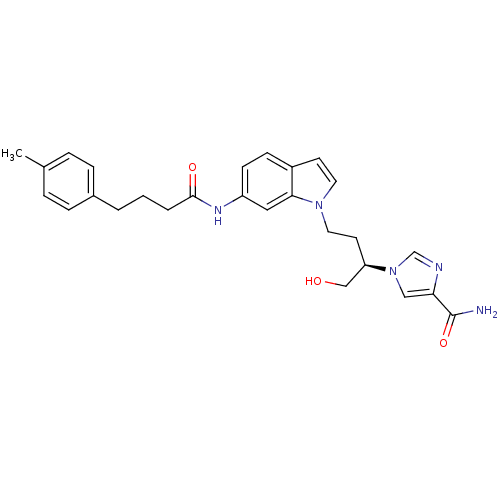
(1-[(2R)-1-hydroxy-4-{6-[4-(4-methylphenyl)butanami...)Show SMILES Cc1ccc(CCCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)cc1 |r| Show InChI InChI=1S/C27H31N5O3/c1-19-5-7-20(8-6-19)3-2-4-26(34)30-22-10-9-21-11-13-31(25(21)15-22)14-12-23(17-33)32-16-24(27(28)35)29-18-32/h5-11,13,15-16,18,23,33H,2-4,12,14,17H2,1H3,(H2,28,35)(H,30,34)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | -10.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22926
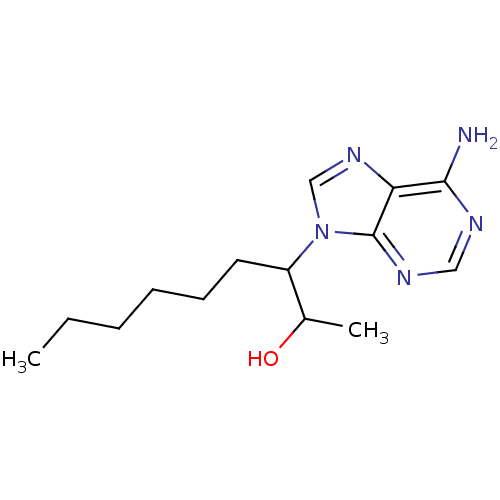
(3-(6-amino-9H-purin-9-yl)nonan-2-ol | EHNA | Eryth...)Show InChI InChI=1S/C14H23N5O/c1-3-4-5-6-7-11(10(2)20)19-9-18-12-13(15)16-8-17-14(12)19/h8-11,20H,3-7H2,1-2H3,(H2,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 37 | -10.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
Bioorg Med Chem Lett 13: 1115-8 (2003)
Article DOI: 10.1016/S0960-894X(03)00026-X
BindingDB Entry DOI: 10.7270/Q29Z936D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22933

(1-[(2R)-1-hydroxy-4-{6-[3-(4-methylphenyl)propanam...)Show SMILES Cc1ccc(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)cc1 |r| Show InChI InChI=1S/C26H29N5O3/c1-18-2-4-19(5-3-18)6-9-25(33)29-21-8-7-20-10-12-30(24(20)14-21)13-11-22(16-32)31-15-23(26(27)34)28-17-31/h2-5,7-8,10,12,14-15,17,22,32H,6,9,11,13,16H2,1H3,(H2,27,34)(H,29,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | -10.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50335313
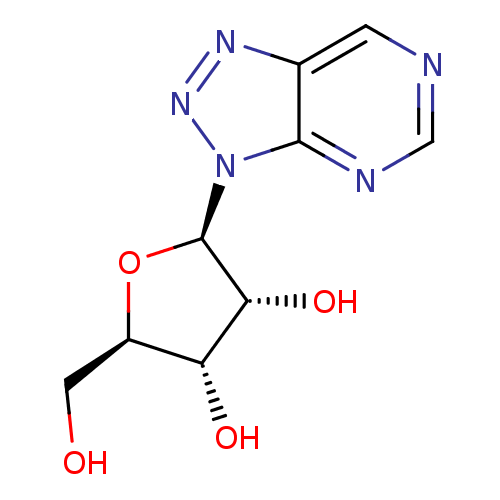
(8-aza-nebularine | CHEMBL1651380)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1nnc2cncnc12 |r| Show InChI InChI=1S/C9H11N5O4/c15-2-5-6(16)7(17)9(18-5)14-8-4(12-13-14)1-10-3-11-8/h1,3,5-7,9,15-17H,2H2/t5-,6-,7-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
J Med Chem 54: 107-21 (2011)
Article DOI: 10.1021/jm101286g
BindingDB Entry DOI: 10.7270/Q2K64K1Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22940

(1-[(2R)-4-[6-(hexyloxy)-1H-indol-1-yl]-1-hydroxybu...)Show SMILES CCCCCCOc1ccc2ccn(CC[C@H](CO)n3cnc(c3)C(N)=O)c2c1 |r| Show InChI InChI=1S/C22H30N4O3/c1-2-3-4-5-12-29-19-7-6-17-8-10-25(21(17)13-19)11-9-18(15-27)26-14-20(22(23)28)24-16-26/h6-8,10,13-14,16,18,27H,2-5,9,11-12,15H2,1H3,(H2,23,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | -9.80 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22934
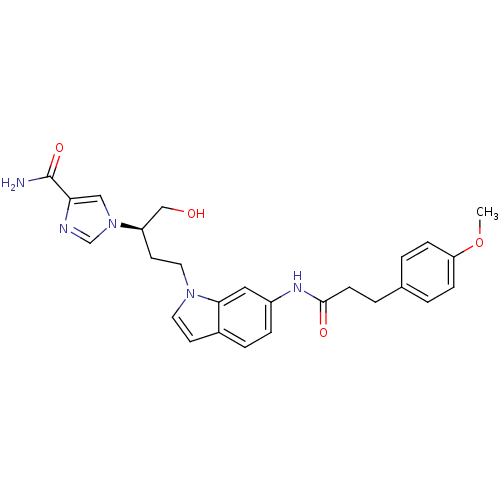
(1-[(2R)-1-hydroxy-4-{6-[3-(4-methoxyphenyl)propana...)Show SMILES COc1ccc(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)cc1 |r| Show InChI InChI=1S/C26H29N5O4/c1-35-22-7-2-18(3-8-22)4-9-25(33)29-20-6-5-19-10-12-30(24(19)14-20)13-11-21(16-32)31-15-23(26(27)34)28-17-31/h2-3,5-8,10,12,14-15,17,21,32H,4,9,11,13,16H2,1H3,(H2,27,34)(H,29,33)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | -9.78 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50404651
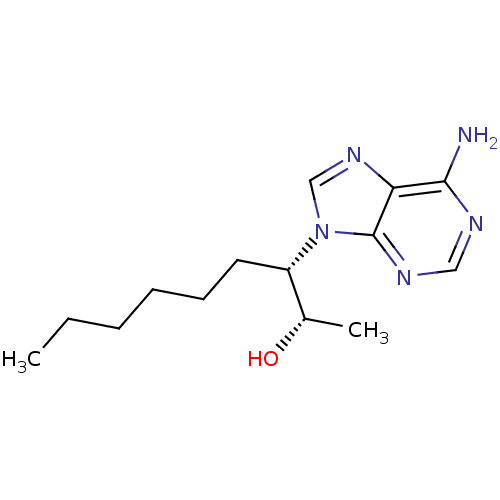
(CHEMBL1555103)Show InChI InChI=1S/C14H23N5O/c1-3-4-5-6-7-11(10(2)20)19-9-18-12-13(15)16-8-17-14(12)19/h8-11,20H,3-7H2,1-2H3,(H2,15,16,17)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22932
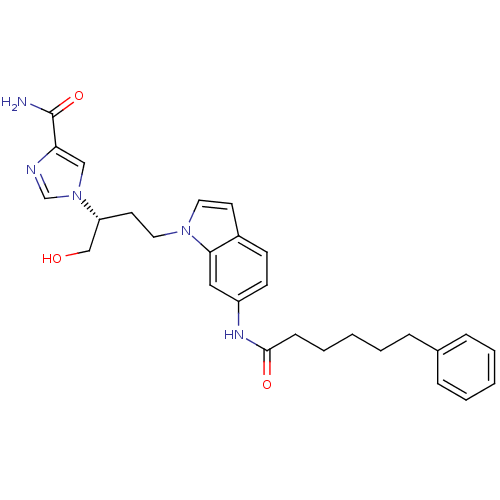
(1-[(2R)-1-hydroxy-4-[6-(6-phenylhexanamido)-1H-ind...)Show SMILES NC(=O)c1cn(cn1)[C@@H](CO)CCn1ccc2ccc(NC(=O)CCCCCc3ccccc3)cc12 |r| Show InChI InChI=1S/C28H33N5O3/c29-28(36)25-18-33(20-30-25)24(19-34)14-16-32-15-13-22-11-12-23(17-26(22)32)31-27(35)10-6-2-5-9-21-7-3-1-4-8-21/h1,3-4,7-8,11-13,15,17-18,20,24,34H,2,5-6,9-10,14,16,19H2,(H2,29,36)(H,31,35)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | -9.50 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 3730-43 (2004)
Article DOI: 10.1021/jm0306374
BindingDB Entry DOI: 10.7270/Q2668BG0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data