 Found 44 hits Enz. Inhib. hit(s) with all data for entry = 50002747
Found 44 hits Enz. Inhib. hit(s) with all data for entry = 50002747 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222244
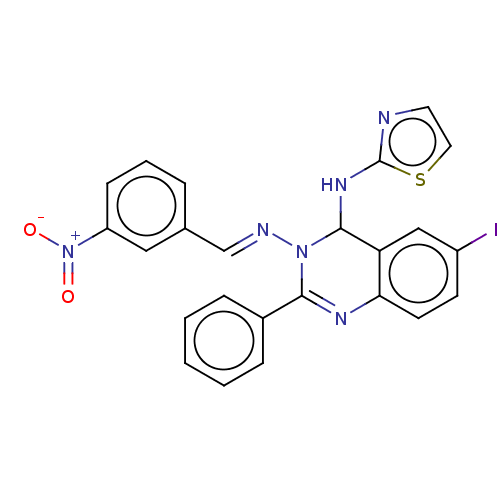
(6-Iodo-N3-(3-nitrobenzylidene)-2-phenyl-N4-(thiazo...)Show SMILES [O-][N+](=O)c1cccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)c1 |c:27| Show InChI InChI=1S/C24H17IN6O2S/c25-18-9-10-21-20(14-18)23(29-24-26-11-12-34-24)30(22(28-21)17-6-2-1-3-7-17)27-15-16-5-4-8-19(13-16)31(32)33/h1-15,23H,(H,26,29)/b27-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using H-Gly-Pro-AMC as substrate |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462429
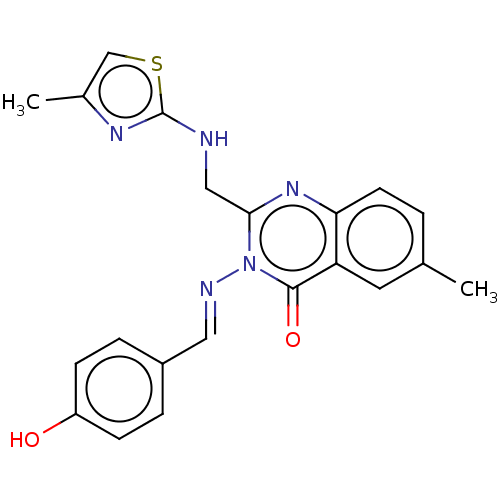
(CHEMBL4247480)Show SMILES Cc1csc(NCc2nc3ccc(C)cc3c(=O)n2\N=C\c2ccc(O)cc2)n1 Show InChI InChI=1S/C21H19N5O2S/c1-13-3-8-18-17(9-13)20(28)26(23-10-15-4-6-16(27)7-5-15)19(25-18)11-22-21-24-14(2)12-29-21/h3-10,12,27H,11H2,1-2H3,(H,22,24)/b23-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using H-Gly-Pro-AMC as substrate |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222254

(6-Iodo-N4-(4-methylthiazol-2-yl)-N3-(3-nitrobenzyl...)Show SMILES Cc1csc(NC2N(\N=C\c3cccc(c3)[N+]([O-])=O)C(=Nc3ccc(I)cc23)c2ccccc2)n1 |c:20| Show InChI InChI=1S/C25H19IN6O2S/c1-16-15-35-25(28-16)30-24-21-13-19(26)10-11-22(21)29-23(18-7-3-2-4-8-18)31(24)27-14-17-6-5-9-20(12-17)32(33)34/h2-15,24H,1H3,(H,28,30)/b27-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using H-Gly-Pro-AMC as substrate |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222239
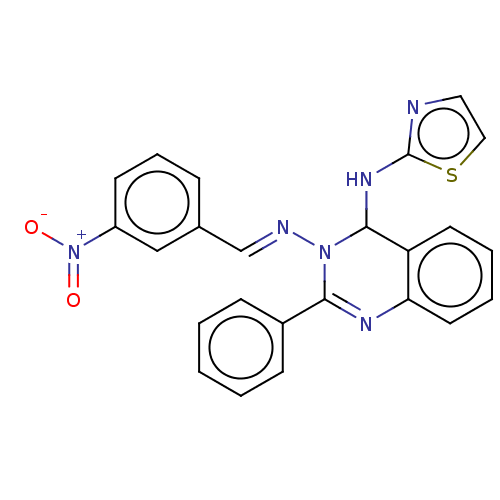
(N3-(3-Nitrobenzylidene)-2-phenyl-N4-(thiazol-2-yl)...)Show SMILES [O-][N+](=O)c1cccc(\C=N\N2C(Nc3nccs3)c3ccccc3N=C2c2ccccc2)c1 |c:26| Show InChI InChI=1S/C24H18N6O2S/c31-30(32)19-10-6-7-17(15-19)16-26-29-22(18-8-2-1-3-9-18)27-21-12-5-4-11-20(21)23(29)28-24-25-13-14-33-24/h1-16,23H,(H,25,28)/b26-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using H-Gly-Pro-AMC as substrate |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM222245
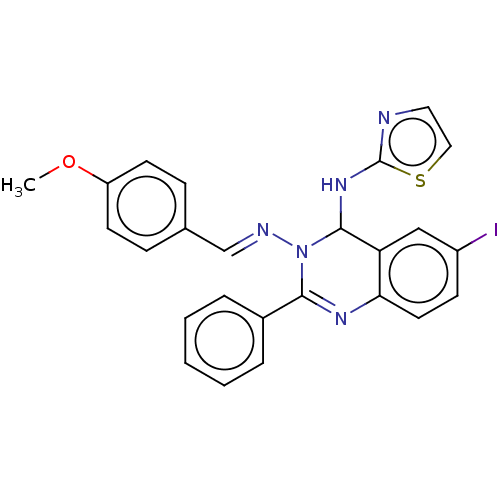
(6-Iodo-N3-(4-methoxybenzylidene)-2-phenyl-N4-(thia...)Show SMILES COc1ccc(\C=N\N2C(Nc3nccs3)c3cc(I)ccc3N=C2c2ccccc2)cc1 |c:25| Show InChI InChI=1S/C25H20IN5OS/c1-32-20-10-7-17(8-11-20)16-28-31-23(18-5-3-2-4-6-18)29-22-12-9-19(26)15-21(22)24(31)30-25-27-13-14-33-25/h2-16,24H,1H3,(H,27,30)/b28-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using H-Gly-Pro-AMC as substrate |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462425

(CHEMBL4241283)Show InChI InChI=1S/C10H20N2OS/c1-10(2,3)8(11)9(13)12-4-6-14-7-5-12/h8H,4-7,11H2,1-3H3/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462419

(CHEMBL4250956)Show InChI InChI=1S/C10H20N2OS/c1-8(2)7-9(11)10(13)12-3-5-14-6-4-12/h8-9H,3-7,11H2,1-2H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462426

(CHEMBL4241366)Show InChI InChI=1S/C10H20N2OS/c1-3-8(2)9(11)10(13)12-4-6-14-7-5-12/h8-9H,3-7,11H2,1-2H3/t8?,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 using Gly-Pro-AMC as substrate after 1 hr by fluorescence assay |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462415
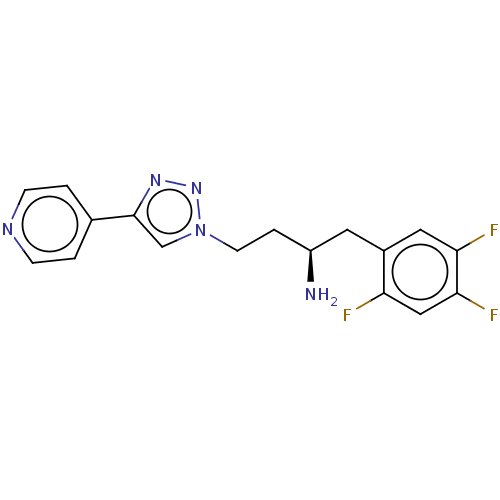
(CHEMBL4250492)Show SMILES N[C@@H](CCn1cc(nn1)-c1ccncc1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C17H16F3N5/c18-14-9-16(20)15(19)8-12(14)7-13(21)3-6-25-10-17(23-24-25)11-1-4-22-5-2-11/h1-2,4-5,8-10,13H,3,6-7,21H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 using Gly-Pro-AMC as substrate after 1 hr by fluorescence assay |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462418

(CHEMBL4246674)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-c3ccccc3C#N)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6](-[#7])-[#6]-1 Show InChI InChI=1S/C24H29N7O2/c1-16(2)10-12-30-20-21(27-23(30)29-11-6-9-19(26)15-29)28(3)24(33)31(22(20)32)14-18-8-5-4-7-17(18)13-25/h4-5,7-8,10,19H,6,9,11-12,14-15,26H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 pre-incubated for 30 mins before Gly-Pro-Aminomethylcoumarin substrate addition |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462427

(CHEMBL4250558)Show SMILES Clc1ccc(NC(=O)CNC(=O)C2CCCN(C2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C20H22ClN3O4S/c21-16-8-10-17(11-9-16)23-19(25)13-22-20(26)15-5-4-12-24(14-15)29(27,28)18-6-2-1-3-7-18/h1-3,6-11,15H,4-5,12-14H2,(H,22,26)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50028962
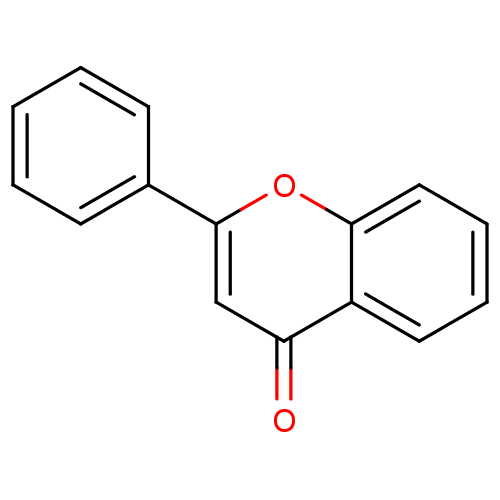
(CHEMBL275638 | flavone)Show InChI InChI=1S/C15H10O2/c16-13-10-15(11-6-2-1-3-7-11)17-14-9-5-4-8-12(13)14/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462430

(CHEMBL4246222)Show SMILES NCc1c(-c2ccc(Cl)cc2Cl)n(CS(=O)=O)c2ccccc12 |(34.01,-17.35,;35.05,-18.49,;34.58,-19.96,;35.5,-21.2,;37.03,-21.19,;37.81,-22.52,;39.35,-22.51,;40.11,-21.17,;41.65,-21.16,;39.32,-19.84,;37.79,-19.86,;37.01,-18.53,;34.6,-22.45,;35.08,-23.91,;34.06,-25.06,;34.54,-26.52,;32.55,-24.75,;33.13,-21.98,;31.79,-22.76,;30.46,-21.99,;30.46,-20.45,;31.79,-19.68,;33.12,-20.44,)| Show InChI InChI=1S/C16H14Cl2N2O2S/c17-10-5-6-12(14(18)7-10)16-13(8-19)11-3-1-2-4-15(11)20(16)9-23(21)22/h1-7,23H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM23419
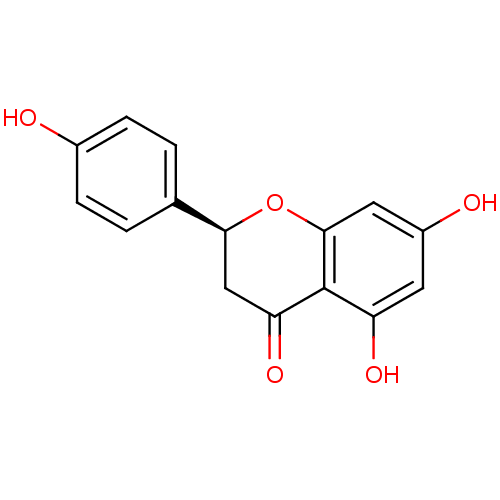
((2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro...)Show SMILES Oc1ccc(cc1)[C@@H]1CC(=O)c2c(O)cc(O)cc2O1 |r| Show InChI InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM23418
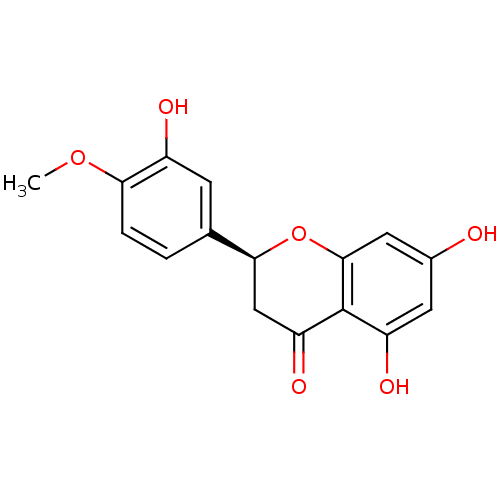
((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...)Show SMILES COc1ccc(cc1O)[C@@H]1CC(=O)c2c(O)cc(O)cc2O1 |r| Show InChI InChI=1S/C16H14O6/c1-21-13-3-2-8(4-10(13)18)14-7-12(20)16-11(19)5-9(17)6-15(16)22-14/h2-6,14,17-19H,7H2,1H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50347135
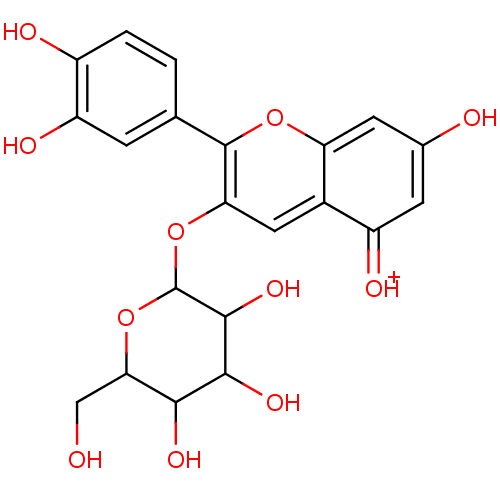
(Cyanidin-3-glucoside (M8) | KUROMANIN)Show SMILES OCC1OC(Oc2cc3c(cc(O)cc3=[OH+])oc2-c2ccc(O)c(O)c2)C(O)C(O)C1O Show InChI InChI=1S/C21H20O11/c22-7-16-17(27)18(28)19(29)21(32-16)31-15-6-10-12(25)4-9(23)5-14(10)30-20(15)8-1-2-11(24)13(26)3-8/h1-6,16-19,21-24,26-29H,7H2/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50049394
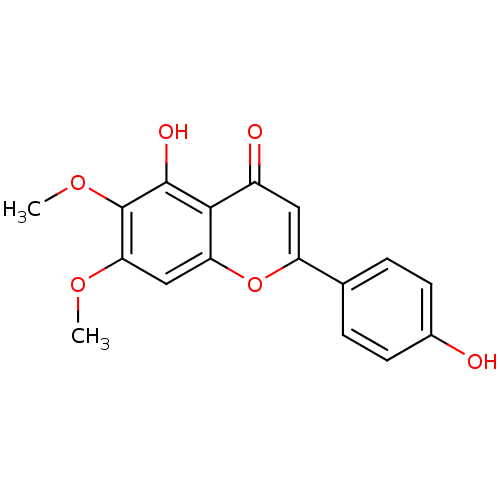
(5,4'-Dihydroxy-6,7-dimethoxyflavone | 5-Hydroxy-2-...)Show InChI InChI=1S/C17H14O6/c1-21-14-8-13-15(16(20)17(14)22-2)11(19)7-12(23-13)9-3-5-10(18)6-4-9/h3-8,18,20H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 after 30 mins by luminescence assay |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM19459

(5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50049395
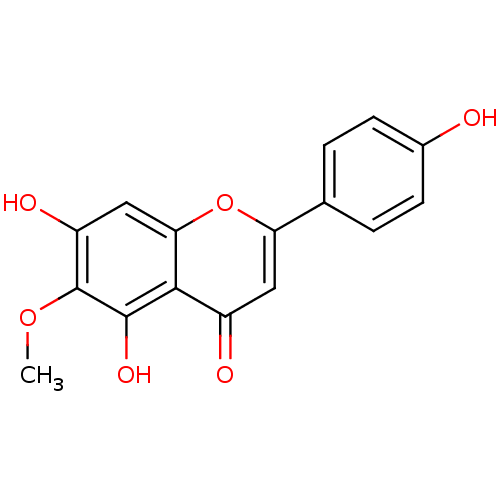
(5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...)Show InChI InChI=1S/C16H12O6/c1-21-16-11(19)7-13-14(15(16)20)10(18)6-12(22-13)8-2-4-9(17)5-3-8/h2-7,17,19-20H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 after 30 mins by luminescence assay |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462416

(CHEMBL4249750)Show SMILES FC1(F)CCN(C1)C(=O)[C@@H]1C[C@@H](CN1)n1cc(nn1)-c1ccccc1 |r| Show InChI InChI=1S/C17H19F2N5O/c18-17(19)6-7-23(11-17)16(25)14-8-13(9-20-14)24-10-15(21-22-24)12-4-2-1-3-5-12/h1-5,10,13-14,20H,6-9,11H2/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in baculovirus expression system using Ala-Pro-AMC as substrate by fluorometric analysis |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462428

(CHEMBL4241199)Show SMILES COC(=O)c1cn(nn1)[C@@H]1CN[C@@H](C1)C(=O)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C13H17F2N5O3/c1-23-12(22)10-6-20(18-17-10)8-4-9(16-5-8)11(21)19-3-2-13(14,15)7-19/h6,8-9,16H,2-5,7H2,1H3/t8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in baculovirus expression system using Ala-Pro-AMC as substrate by fluorometric analysis |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462420

(CHEMBL4240687)Show SMILES Cc1cc(ccc1F)-c1cn(nn1)[C@@H]1CN[C@@H](C1)C(=O)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C18H20F3N5O/c1-11-6-12(2-3-14(11)19)16-9-26(24-23-16)13-7-15(22-8-13)17(27)25-5-4-18(20,21)10-25/h2-3,6,9,13,15,22H,4-5,7-8,10H2,1H3/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 expressed in baculovirus expression system using Ala-Pro-AMC as substrate by fluorometric analysis |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462423
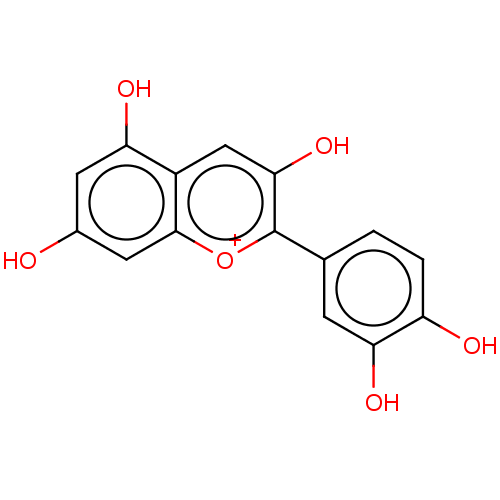
(Cyanidin Chloride)Show SMILES [Cl-].Oc1cc(O)c2cc(O)c([o+]c2c1)-c1ccc(O)c(O)c1 Show InChI InChI=1S/C15H10O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-6H,(H4-,16,17,18,19,20)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276155

(Malvidin Chloride)Show SMILES [Cl-].COc1cc(cc(OC)c1O)-c1[o+]c2cc(O)cc(O)c2cc1O Show InChI InChI=1S/C17H14O7/c1-22-14-3-8(4-15(23-2)16(14)21)17-12(20)7-10-11(19)5-9(18)6-13(10)24-17/h3-7H,1-2H3,(H3-,18,19,20,21)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462433

(CHEMBL4247286)Show InChI InChI=1S/C19H18N4O2/c24-17-10-9-12-5-1-2-6-13(12)15(17)11-20-23-19(25)18-14-7-3-4-8-16(14)21-22-18/h1-2,5-6,9-11,24H,3-4,7-8H2,(H,21,22)(H,23,25)/b20-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 using H-Gly-Pro-AMC as substrate preincubated for 15 mins followed by substrate addition measured for 20 mins wi... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM19461
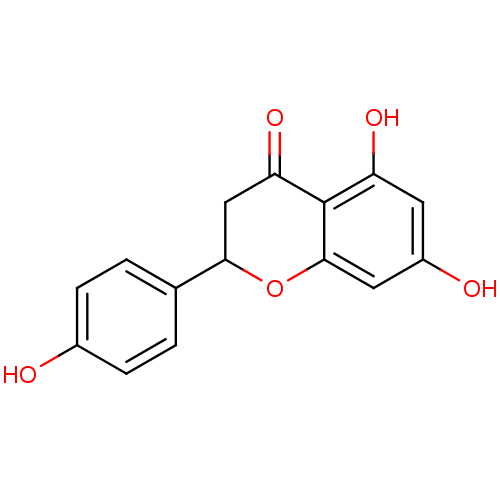
(α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...)Show InChI InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 after 30 mins by luminescence assay |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM4375
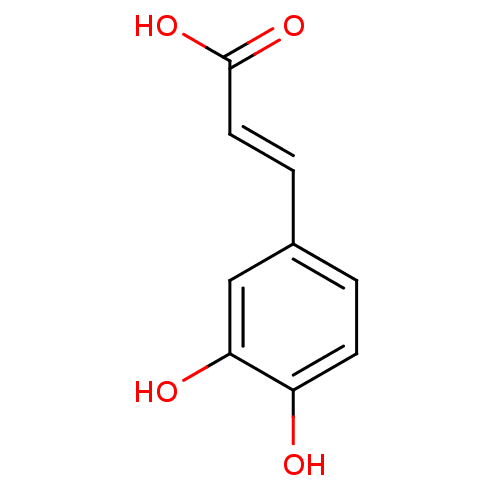
((2E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | (2...)Show InChI InChI=1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462431

(CHEMBL1976036)Show InChI InChI=1S/C19H18N4O/c24-19(18-16-10-3-4-11-17(16)21-22-18)23-20-12-14-8-5-7-13-6-1-2-9-15(13)14/h1-2,5-9,12H,3-4,10-11H2,(H,21,22)(H,23,24)/b20-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 using H-Gly-Pro-AMC as substrate preincubated for 15 mins followed by substrate addition measured for 20 mins wi... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM152800

(1-(2-(4-(7-Chloro-4-quinolyl)piperazin-1-yl)acetyl...)Show InChI InChI=1S/C19H23ClN4O/c20-15-3-4-16-17(13-15)21-6-5-18(16)23-11-9-22(10-12-23)14-19(25)24-7-1-2-8-24/h3-6,13H,1-2,7-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 assessed as hydrolyzing H-Gly-Pro-pNA hydrolysis by measuring p-nitroaniline release after 10 mins by double bea... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50229666
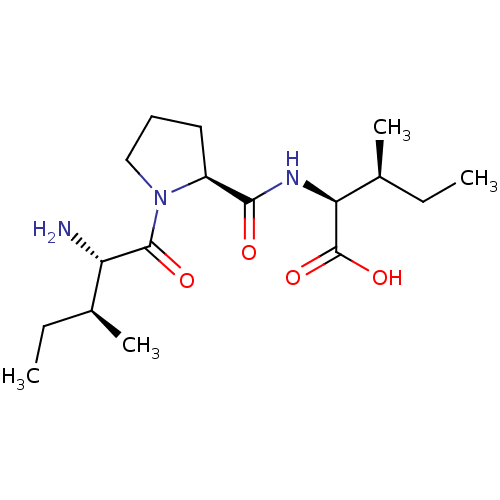
((2S,3S)-2-((S)-1-((2S,3S)-2-amino-3-methylpentanoy...)Show SMILES CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O Show InChI InChI=1S/C17H31N3O4/c1-5-10(3)13(18)16(22)20-9-7-8-12(20)15(21)19-14(17(23)24)11(4)6-2/h10-14H,5-9,18H2,1-4H3,(H,19,21)(H,23,24)/t10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 4.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50085536

(3,4,5-Trihydroxybenzoate, X | 3,4,5-trihydroxybenz...)Show InChI InChI=1S/C7H6O5/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,8-10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462417

(CHEBI:28709 | Eriocitrin)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3cc(O)c4C(=O)C[C@H](Oc4c3)c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H32O15/c1-9-20(32)22(34)24(36)26(39-9)38-8-18-21(33)23(35)25(37)27(42-18)40-11-5-14(30)19-15(31)7-16(41-17(19)6-11)10-2-3-12(28)13(29)4-10/h2-6,9,16,18,20-30,32-37H,7-8H2,1H3/t9-,16-,18+,20-,21+,22+,23-,24+,25+,26+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50462424

(CHEMBL4246951)Show InChI InChI=1S/C12H8BrN5O/c13-11-14-12-16-15-8(6-18(12)17-11)10-5-7-3-1-2-4-9(7)19-10/h1-5H,6H2,(H,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP4 using Gly-Pro-AMC as substrate after 30 mins by fluorescence assay |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50462421

(CHEMBL4242290)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H36N4O5/c1-11(2)9-14(21)17(25)23-15(10-12(3)4)18(26)22-13(5)19(27)24-8-6-7-16(24)20(28)29/h11-16H,6-10,21H2,1-5H3,(H,22,26)(H,23,25)(H,28,29)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney DPP4 |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50462432

(CHEMBL4241845)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCSC)C(C)C)C(O)=O |r| Show InChI InChI=1S/C31H51N9O10S/c1-7-16(4)25(31(49)50)40-29(47)20(10-18-12-33-14-35-18)37-28(46)21(11-23(42)43)38-30(48)24(15(2)3)39-22(41)13-34-26(44)17(5)36-27(45)19(32)8-9-51-6/h12,14-17,19-21,24-25H,7-11,13,32H2,1-6H3,(H,33,35)(H,34,44)(H,36,45)(H,37,46)(H,38,48)(H,39,41)(H,40,47)(H,42,43)(H,49,50)/t16-,17-,19-,20-,21-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney DPP4 |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50462422

(CHEMBL4238143)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H36N4O5/c1-6-12(4)16(21)18(26)23-14(10-11(2)3)17(25)22-13(5)19(27)24-9-7-8-15(24)20(28)29/h11-16H,6-10,21H2,1-5H3,(H,22,25)(H,23,26)(H,28,29)/t12-,13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney DPP4 |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50038066
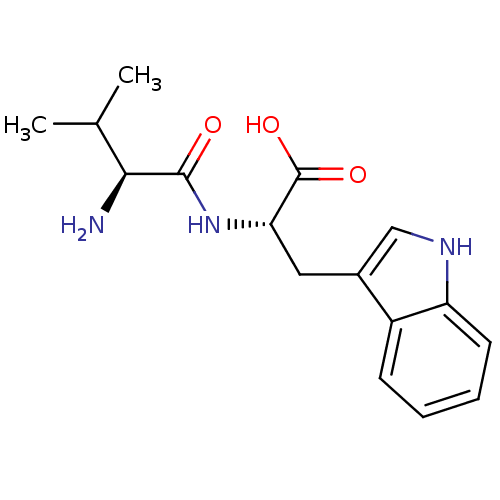
((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-pNA as substrate after 60 mins |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data