

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101871 (3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101882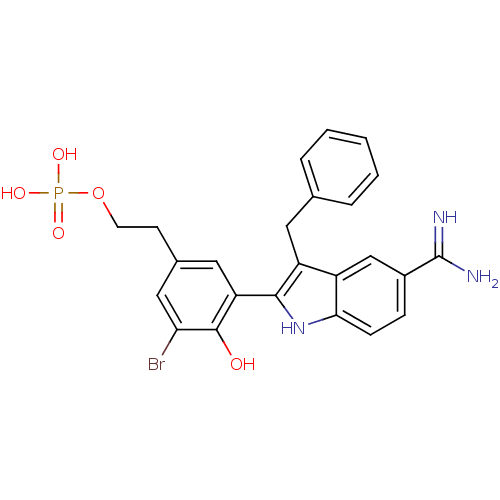 (CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101881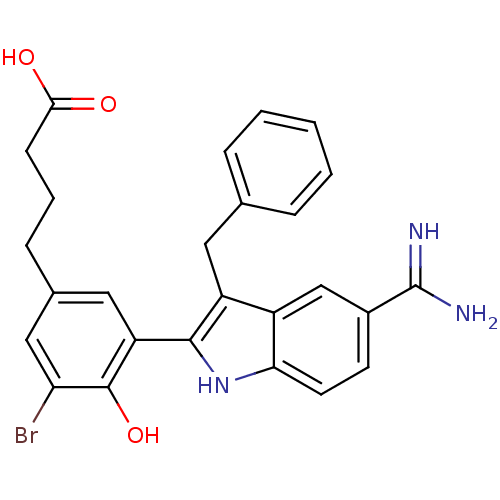 (4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101885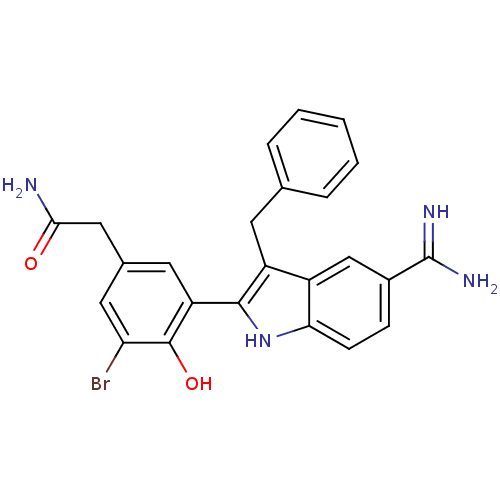 (2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101883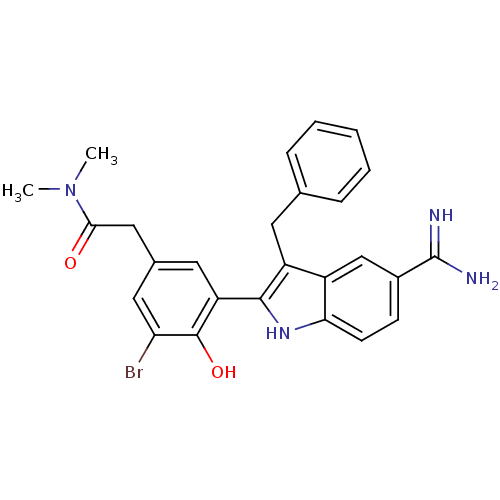 (2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101880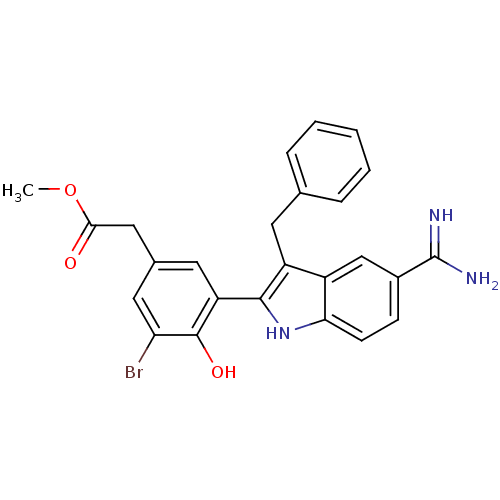 (CHEMBL416127 | [3-(3-Benzyl-5-carbamimidoyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101879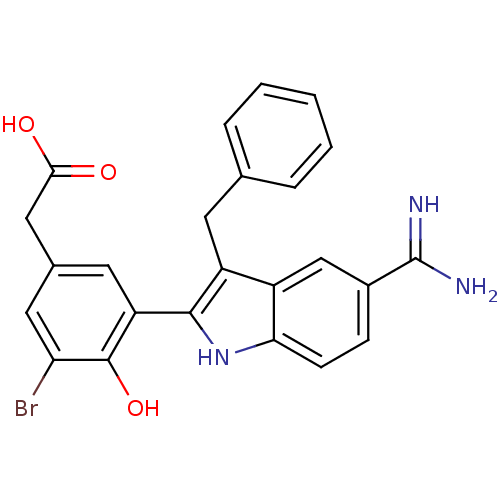 (CHEMBL50924 | [3-(3-Benzyl-5-carbamimidoyl-1H-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101870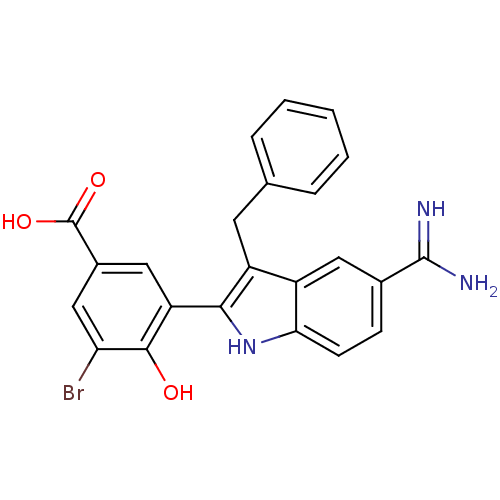 (3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101869 (2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101867 (3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101878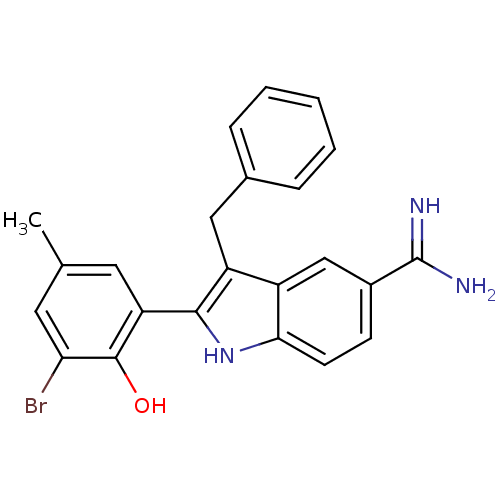 (3-Benzyl-2-(3-bromo-2-hydroxy-5-methyl-phenyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101868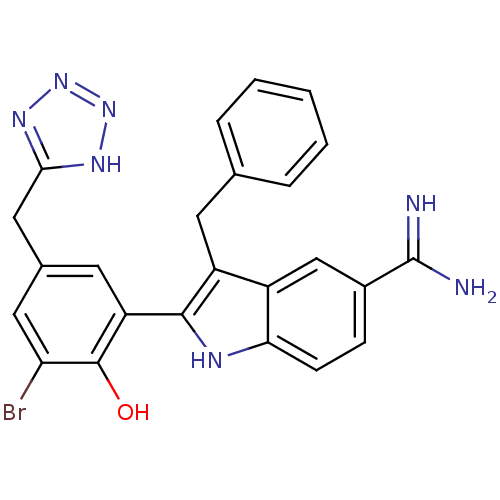 (3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101885 (2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101881 (4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101883 (2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101877 (3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101880 (CHEMBL416127 | [3-(3-Benzyl-5-carbamimidoyl-1H-ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101873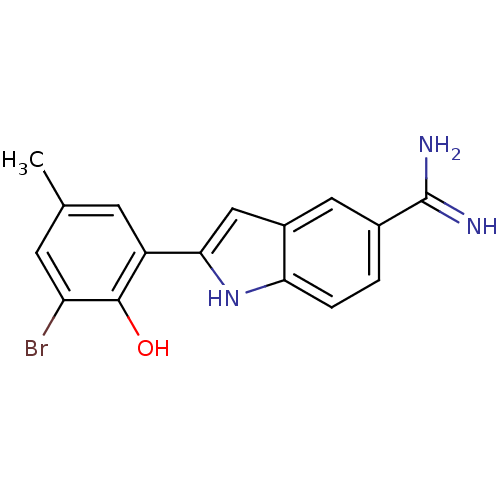 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101866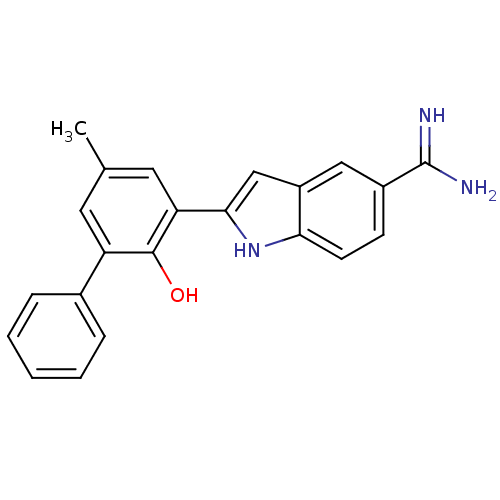 (2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101873 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101871 (3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101876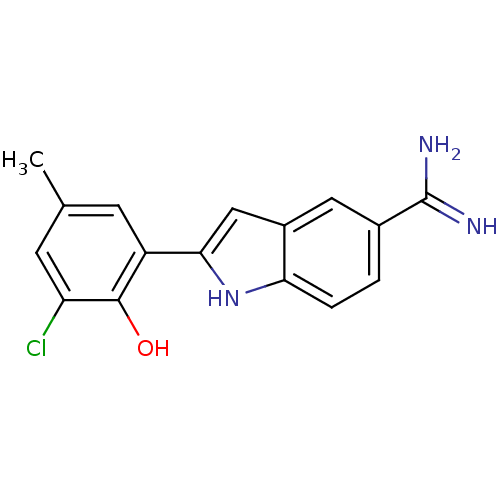 (2-(3-Chloro-2-hydroxy-5-methyl-phenyl)-1H-indole-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101876 (2-(3-Chloro-2-hydroxy-5-methyl-phenyl)-1H-indole-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101878 (3-Benzyl-2-(3-bromo-2-hydroxy-5-methyl-phenyl)-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101881 (4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101871 (3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101870 (3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101882 (CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101870 (3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101867 (3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101868 (3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101869 (2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101874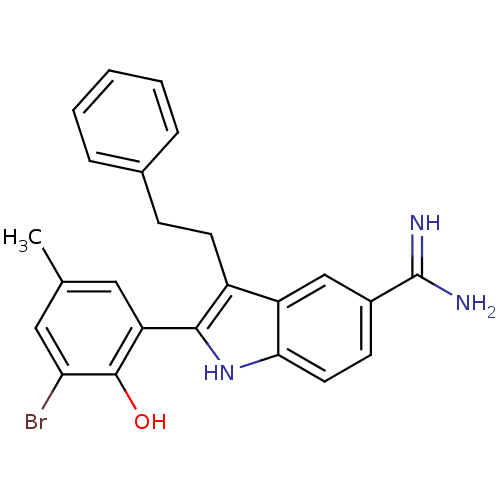 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-3-phenethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101873 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101882 (CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101879 (CHEMBL50924 | [3-(3-Benzyl-5-carbamimidoyl-1H-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50101870 (3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101868 (3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101876 (2-(3-Chloro-2-hydroxy-5-methyl-phenyl)-1H-indole-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101867 (3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50101871 (3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50101881 (4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101866 (2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50101873 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50101876 (2-(3-Chloro-2-hydroxy-5-methyl-phenyl)-1H-indole-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101874 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-3-phenethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101869 (2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50101868 (3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101879 (CHEMBL50924 | [3-(3-Benzyl-5-carbamimidoyl-1H-indo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin(fIIa) in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50101876 (2-(3-Chloro-2-hydroxy-5-methyl-phenyl)-1H-indole-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against plasmin in human plasma | Bioorg Med Chem Lett 11: 1797-800 (2001) BindingDB Entry DOI: 10.7270/Q20K27TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |