

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50250399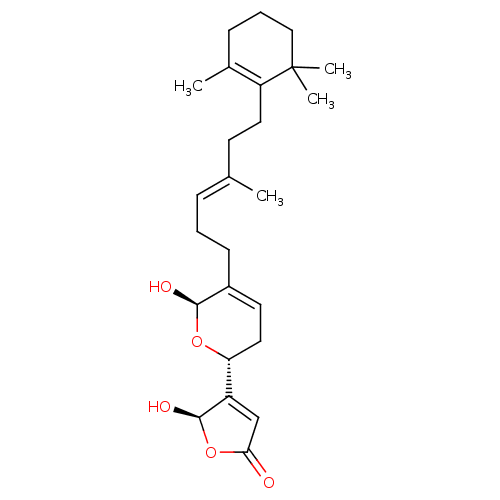 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto per la Chimica di Molecole di Interesse Biologico CNR Curated by ChEMBL | Assay Description Inhibition of human synovial group2 sPLA2 by liquid scintillation counting | J Nat Prod 61: 931-5 (1998) Article DOI: 10.1021/np980122t BindingDB Entry DOI: 10.7270/Q2BK1D8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of human synovial recombinant group 2 secretory phospholipase A2 by liquid scintillation counting | J Nat Prod 64: 612-5 (2001) BindingDB Entry DOI: 10.7270/Q28915MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno Curated by ChEMBL | Assay Description Inhibitory activity against human synovial recombinant Phospholipase enzyme | J Med Chem 41: 3232-8 (1998) Article DOI: 10.1021/jm980027h BindingDB Entry DOI: 10.7270/Q2ZP46SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Rattus norvegicus) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto per la Chimica di Molecole di Interesse Biologico CNR Curated by ChEMBL | Assay Description Inhibition of rat air pouch group2 sPLA2 at 10 uM by liquid scintillation counting | J Nat Prod 61: 931-5 (1998) Article DOI: 10.1021/np980122t BindingDB Entry DOI: 10.7270/Q2BK1D8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto per la Chimica di Molecole di Interesse Biologico CNR Curated by ChEMBL | Assay Description Inhibition of human synovial group2 sPLA2 at 10 uM by liquid scintillation counting | J Nat Prod 61: 931-5 (1998) Article DOI: 10.1021/np980122t BindingDB Entry DOI: 10.7270/Q2BK1D8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). | J Med Chem 39: 5183-91 (1997) Article DOI: 10.1021/jm960437a BindingDB Entry DOI: 10.7270/Q2K64JQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Rattus norvegicus) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of rat secretory Phospholipase A2 (group II). | J Med Chem 39: 5183-91 (1997) Article DOI: 10.1021/jm960437a BindingDB Entry DOI: 10.7270/Q2K64JQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||