

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50208940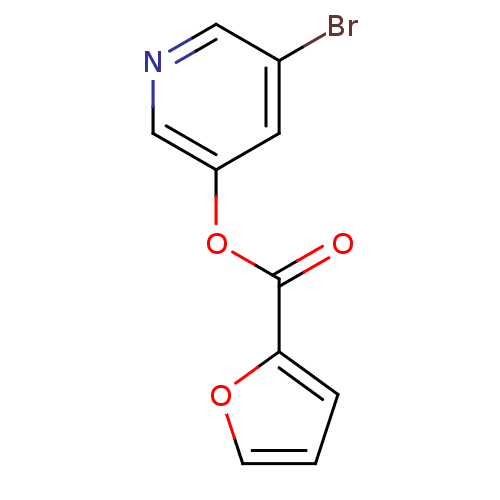 (5-bromo-pyridin-3-yl furan-2-carboxylate | 5-bromo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM32805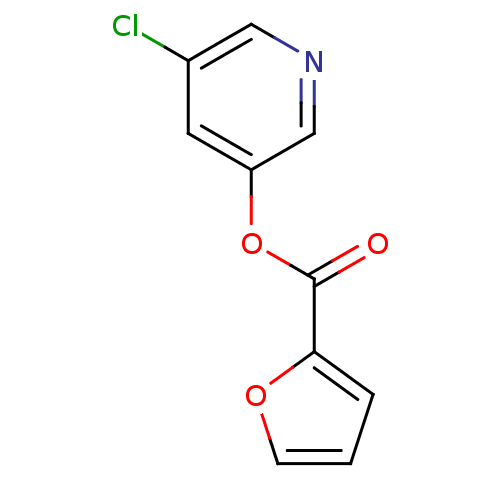 ((5-chloranylpyridin-3-yl) furan-2-carboxylate | (5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50208942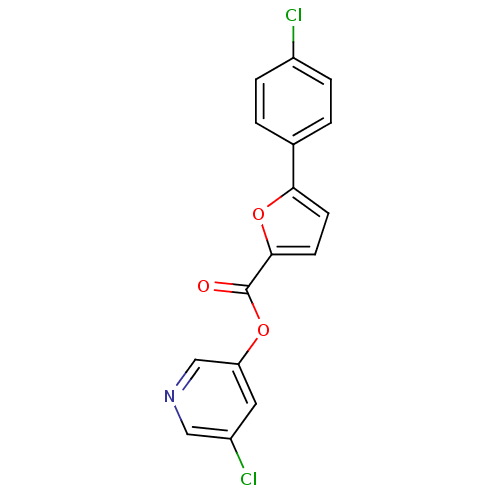 (5-chloropyridin-3-yl 5-(4-chlorophenyl)furan-2-car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50208939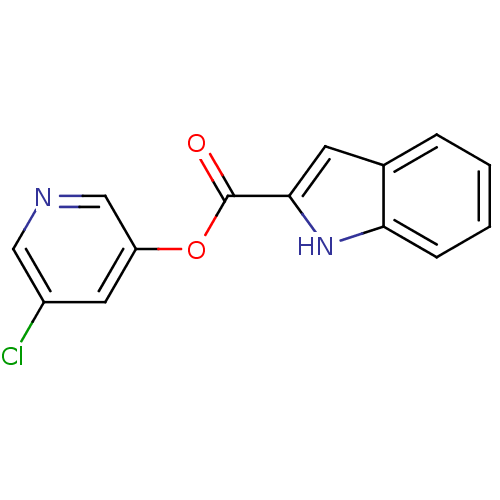 (5-chloro-pyridin-3-yl-1H-indole-2-carboxylate | 5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50208941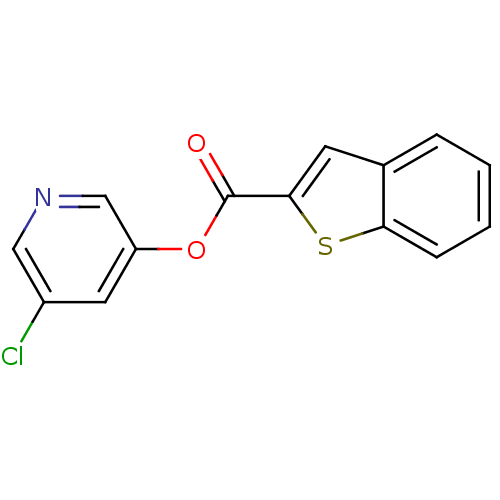 (5-chloropyridin-3-yl benzo[b]thiophene-2-carboxyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582036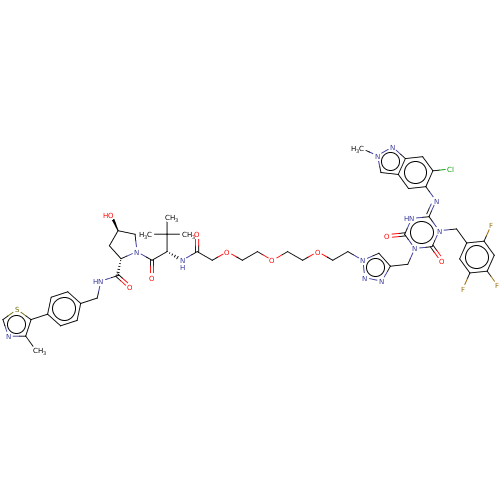 (Preparation of (2S,4R)-1-((S)-2-(tert-butyl)-14-(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582031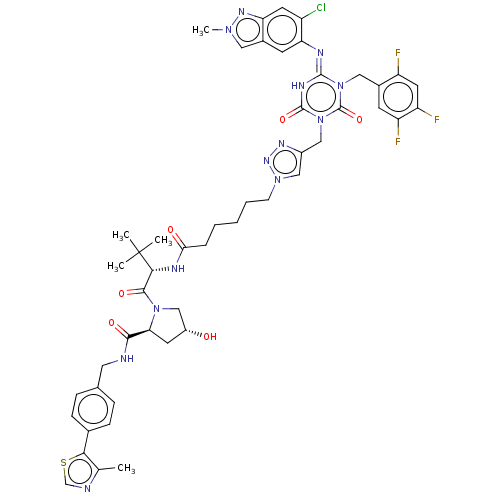 (Preparation of pyrrolidine-2-carboxamide of (2S,4R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582032 (Preparation of (2S,4R)-1-((S)-2-(7-(4-(((E)-4-(6-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582033 (Preparation of (2S,4R)-1-((S)-2-(8-(4-(((E)-4-(6-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582035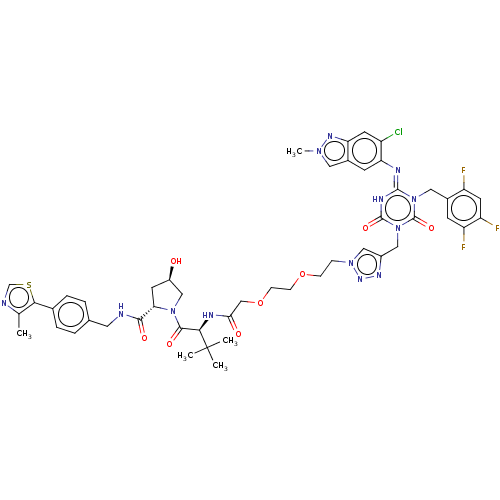 (Preparation of (2S,4R)-1-((S)-2-(2-(2-(2-(4-(((E)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50007791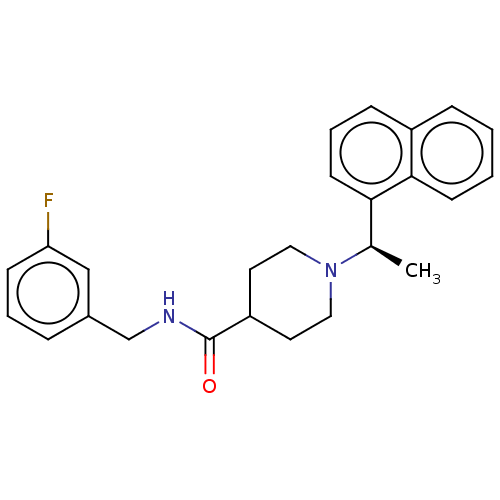 (CHEMBL3233815 | med.21724, Compound 168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50208943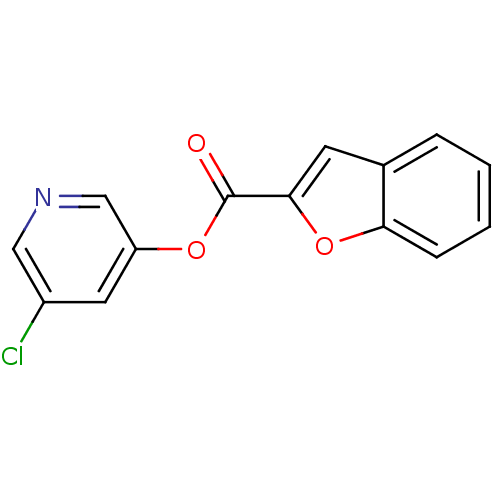 (5-chloropyridin-3-yl benzofuran-2-carboxylate | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50208938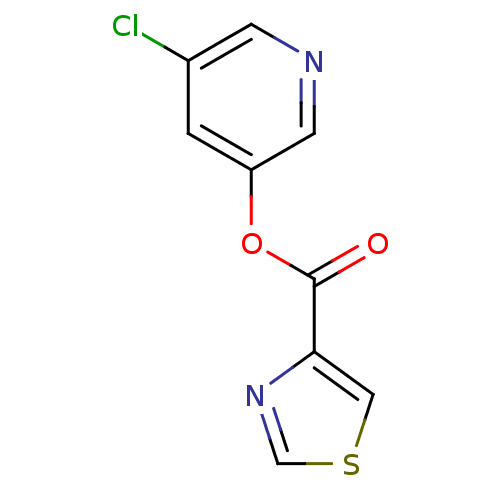 (5-chloropyridin-3-yl thiazole-4-carboxylate | 5-ch...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50007789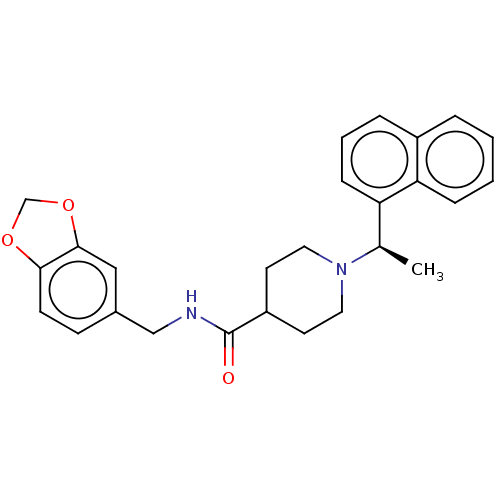 (CHEMBL1173044 | GRL-0667 | N-(2H-1,3-benzodioxol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50208944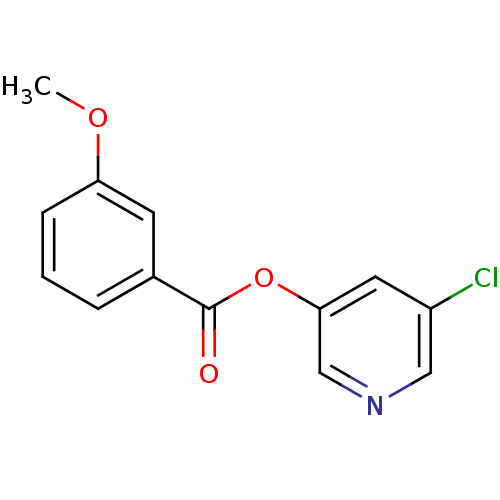 (5-chloropyridin-3-yl 3-methoxybenzoate | 5-chlorop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease | J Med Chem 50: 1850-64 (2007) Article DOI: 10.1021/jm061425k BindingDB Entry DOI: 10.7270/Q2RN37HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50055218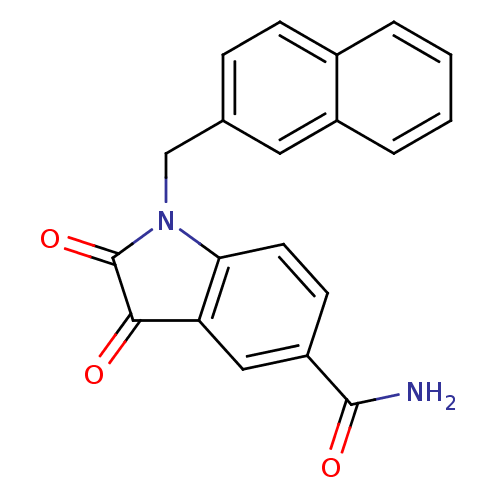 (1-(2-naphthlmethyl) isatin-5-carboxamide | 1-Napht...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of SARS CoV 3C-like protease | J Med Chem 49: 3440-3 (2006) Article DOI: 10.1021/jm0602357 BindingDB Entry DOI: 10.7270/Q2TD9WZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50007788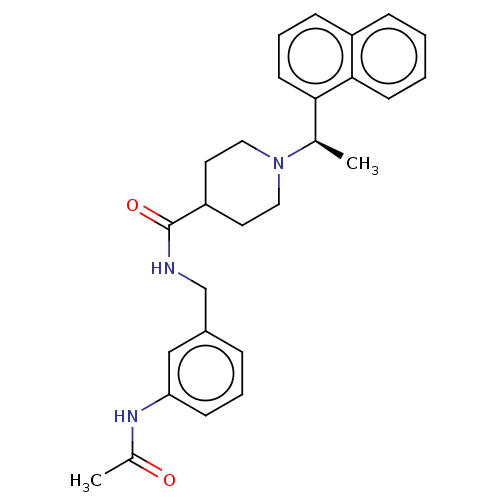 (CHEMBL3233809 | med.21724, Compound 167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM31531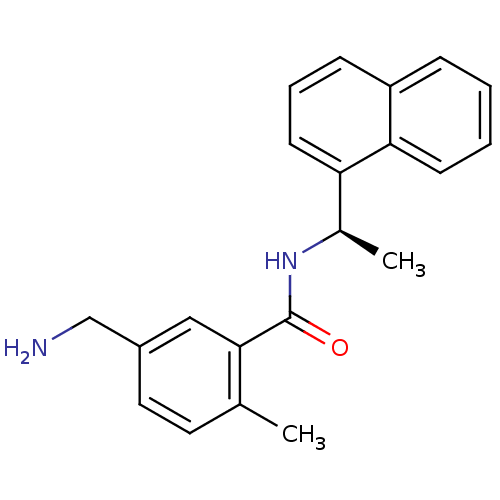 (Substituted Benzamide Derivative, 2 | med.21724, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50007790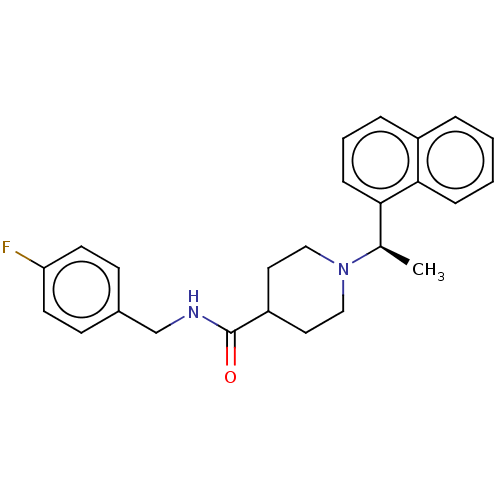 (CHEMBL3233814 | med.21724, Compound 169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582030 (Preparation of (2S,4R)-1-((S)-2-(5-(4-(((E)-4-(6-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582034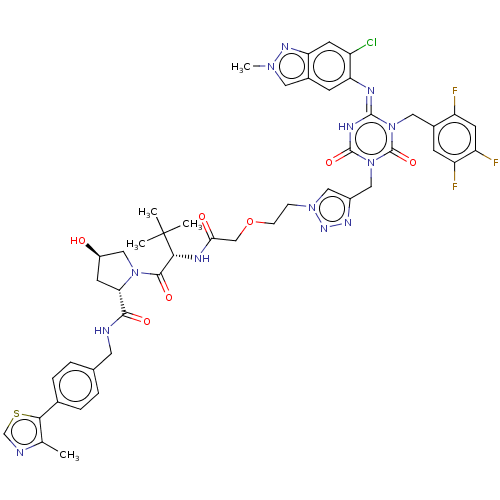 (US11518759, Compound 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582029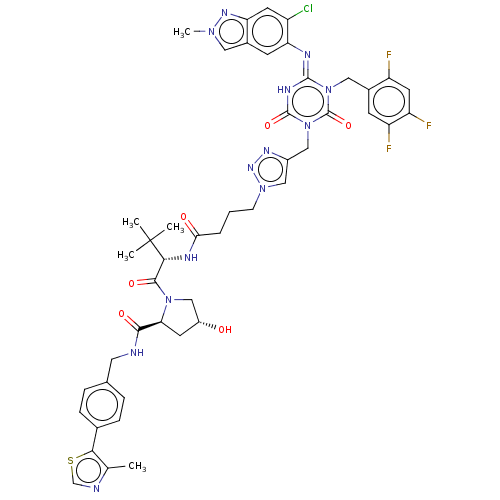 (Preparation of (2S,4R)-1-((S)-2-(4-(4-(((E)-4-(6-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582028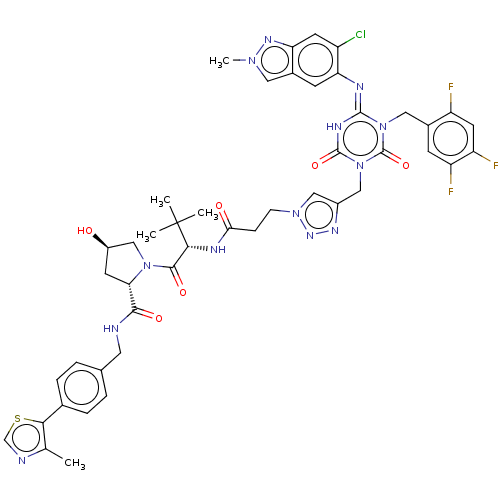 (US11518759, Compound 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0K9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM31523 (Compound 6 | Naphthalene and Benzamide Derivative,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM154574 (N-(2H-1,3-benzodioxol-5-ylmethyl)-1-[(1S)-1-(napht...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM31524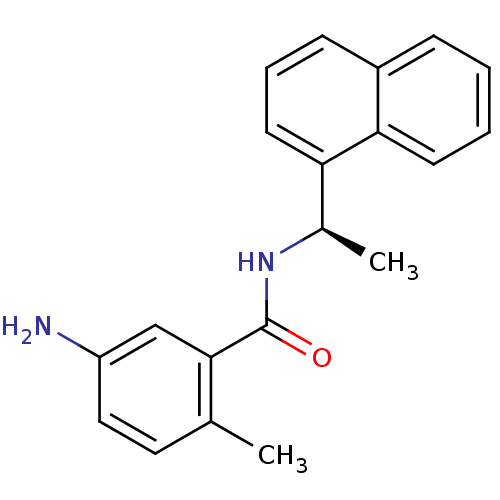 (5-amino-2-methyl-N-[(1R)-1-(naphthalen-1-yl)ethyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM51317 (1,6-dimethylnaphtho[1,2-g][1]benzofuran-10,11-dion...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of SARS-CoV PLpro deubiququitination expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate by fluorescence a... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM53072 ((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate preincubated for 30 mins by fluoresc... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM83922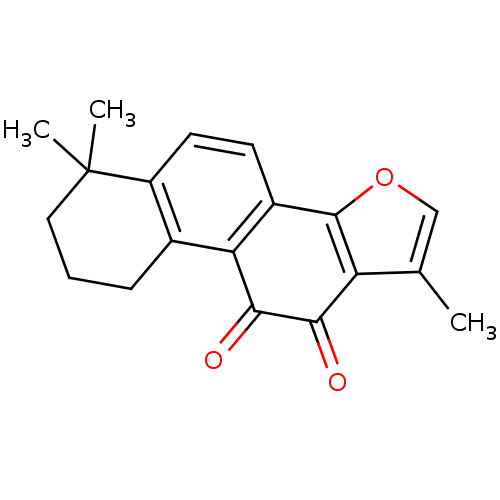 (1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM419127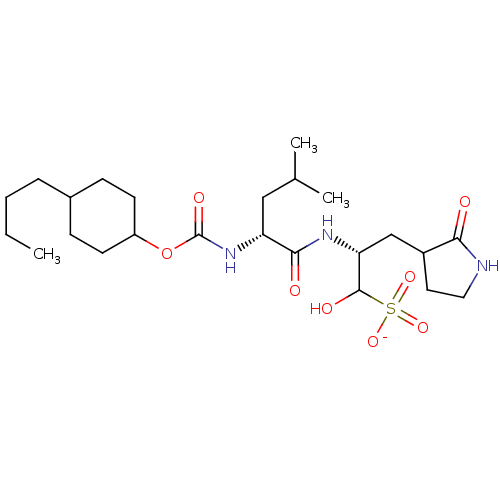 (3CLpro inhibitor 6e) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University | Assay Description The expression and purification of the 3CLpro of MERS-CoV and SARS-CoV were performed by a standard method described previously by our lab (11, 19, 2... | Sci Transl Med 12: (2020) Article DOI: 10.1126/scitranslmed.abc5332 BindingDB Entry DOI: 10.7270/Q21J9D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50167307 (1-Benzo[b]thiophen-2-ylmethyl-5-iodo-1H-indole-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description In vitro inhibitory concentration against SARS coronavirus main protease (SARS CoV 3C-like protease) | Bioorg Med Chem Lett 15: 3058-62 (2005) Article DOI: 10.1016/j.bmcl.2005.04.027 BindingDB Entry DOI: 10.7270/Q2H131JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50167309 (1-Benzo[b]thiophen-2-ylmethyl-7-bromo-1H-indole-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology Curated by ChEMBL | Assay Description In vitro inhibitory concentration SARS coronavirus main protease (SARS CoV 3C-like protease) | Bioorg Med Chem Lett 15: 3058-62 (2005) Article DOI: 10.1016/j.bmcl.2005.04.027 BindingDB Entry DOI: 10.7270/Q2H131JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM419128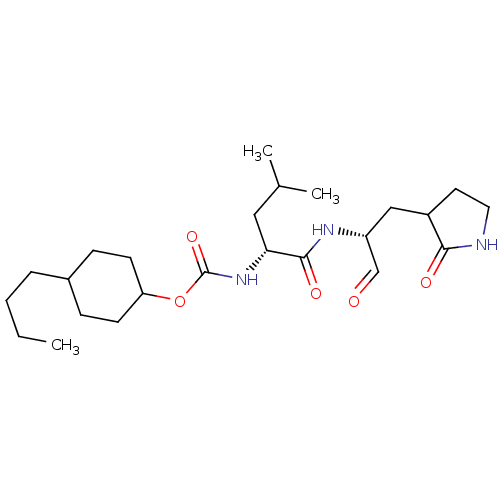 (3CLpro inhibitor 7e) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University | Assay Description The expression and purification of the 3CLpro of MERS-CoV and SARS-CoV were performed by a standard method described previously by our lab (11, 19, 2... | Sci Transl Med 12: (2020) Article DOI: 10.1126/scitranslmed.abc5332 BindingDB Entry DOI: 10.7270/Q21J9D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM419131 (3CLpro inhibitor 6j | 3CLpro inhibitor 7j) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University | Assay Description The expression and purification of the 3CLpro of MERS-CoV and SARS-CoV were performed by a standard method described previously by our lab (11, 19, 2... | Sci Transl Med 12: (2020) Article DOI: 10.1126/scitranslmed.abc5332 BindingDB Entry DOI: 10.7270/Q21J9D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM419131 (3CLpro inhibitor 6j | 3CLpro inhibitor 7j) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University | Assay Description The expression and purification of the 3CLpro of MERS-CoV and SARS-CoV were performed by a standard method described previously by our lab (11, 19, 2... | Sci Transl Med 12: (2020) Article DOI: 10.1126/scitranslmed.abc5332 BindingDB Entry DOI: 10.7270/Q21J9D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM50423877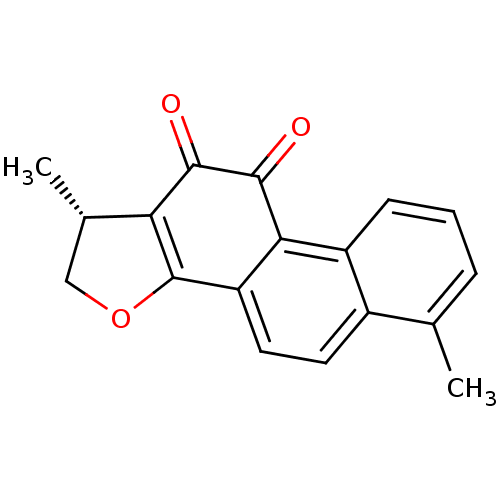 (DIHYDROTANSHINONE | Dihydrotanshinone I | acs.jmed...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of SARS-CoV PLpro deubiququitination expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate by fluorescence a... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM31530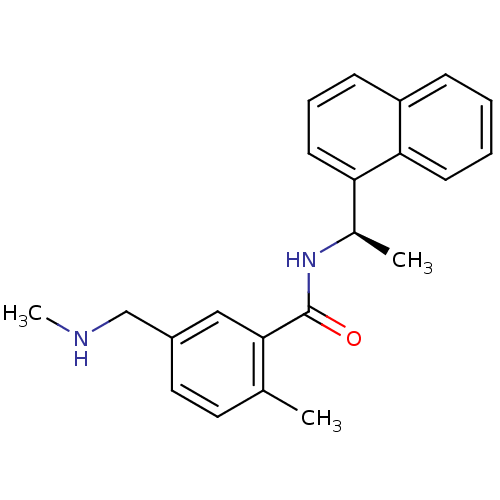 (2-methyl-5-[(methylamino)methyl]-N-[(1R)-1-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM429329 (acs.jmedchem.1c00409_ST.265 | jm5b01461, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | J Med Chem 59: 6595-628 (2016) Article DOI: 10.1021/acs.jmedchem.5b01461 BindingDB Entry DOI: 10.7270/Q2PK0JH1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM83922 (1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate preincubated for 30 mins by fluoresc... | Bioorg Med Chem 20: 5928-35 (2012) Article DOI: 10.1016/j.bmc.2012.07.038 BindingDB Entry DOI: 10.7270/Q2QF8TZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM419126 (3CLpro inhibitor 7c) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University | Assay Description The expression and purification of the 3CLpro of MERS-CoV and SARS-CoV were performed by a standard method described previously by our lab (11, 19, 2... | Sci Transl Med 12: (2020) Article DOI: 10.1126/scitranslmed.abc5332 BindingDB Entry DOI: 10.7270/Q21J9D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM419124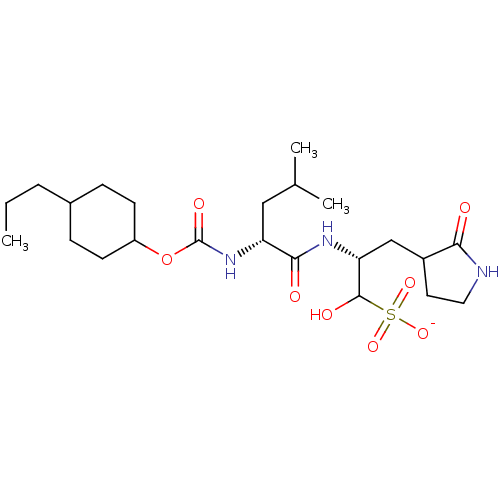 (3CLpro inhibitor 6c) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University | Assay Description The expression and purification of the 3CLpro of MERS-CoV and SARS-CoV were performed by a standard method described previously by our lab (11, 19, 2... | Sci Transl Med 12: (2020) Article DOI: 10.1126/scitranslmed.abc5332 BindingDB Entry DOI: 10.7270/Q21J9D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582764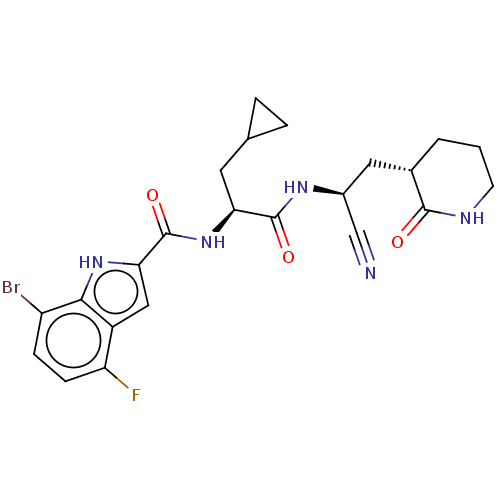 (US11524940, Compound 854) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582765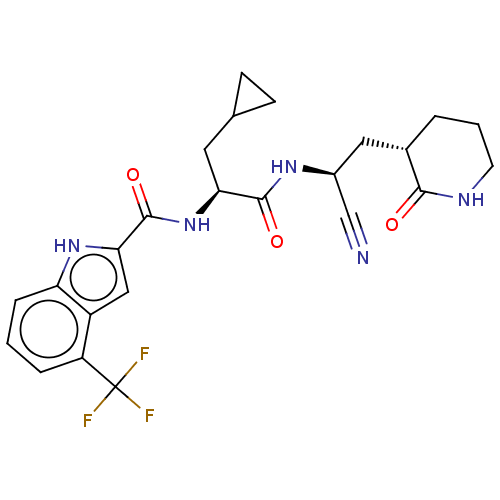 (US11524940, Compound 856) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582766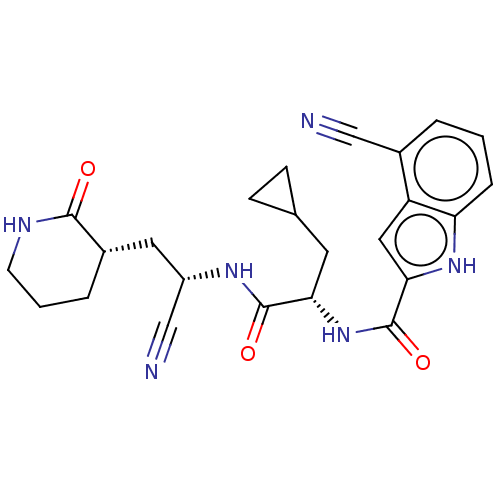 (US11524940, Compound 858) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582767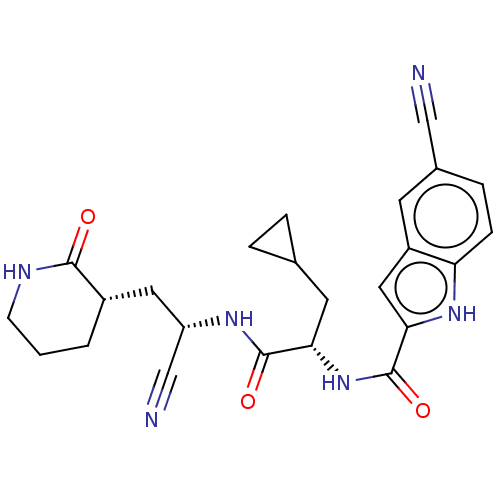 (US11524940, Compound 860) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582768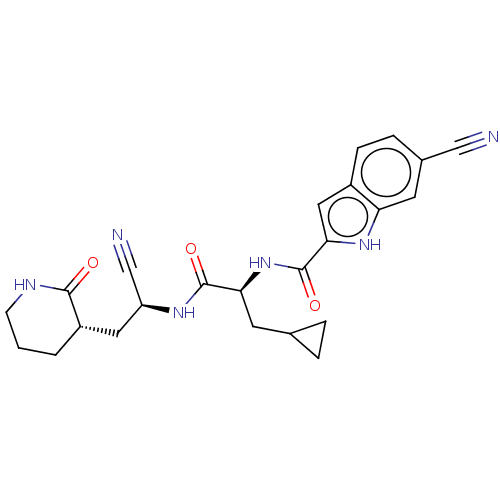 (US11524940, Compound 862) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582769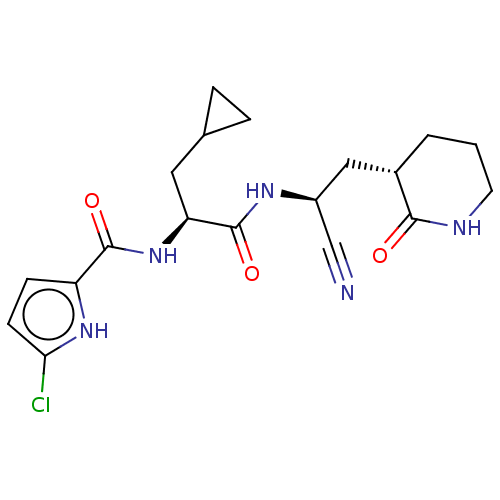 (US11524940, Compound 864) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582770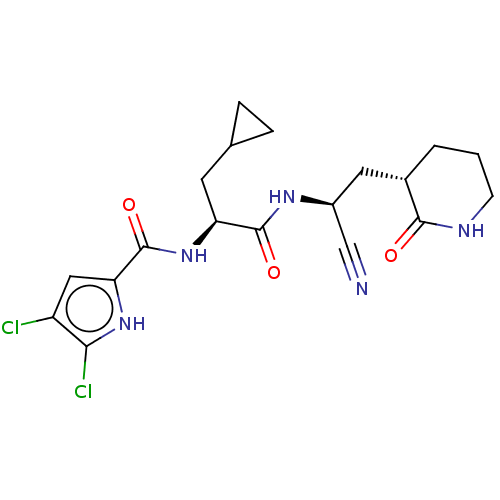 (US11524940, Compound 866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582771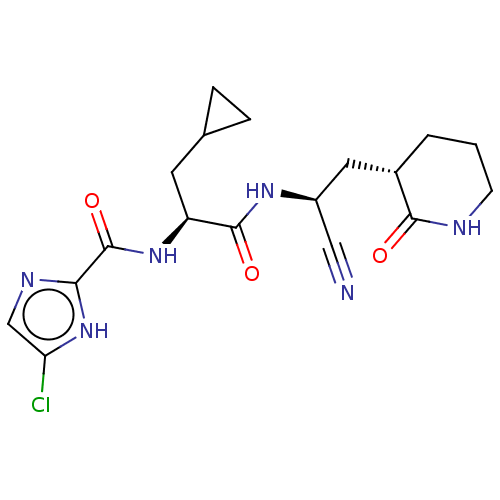 (US11524940, Compound 868) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human SARS coronavirus (SARS-CoV)) | BDBM582773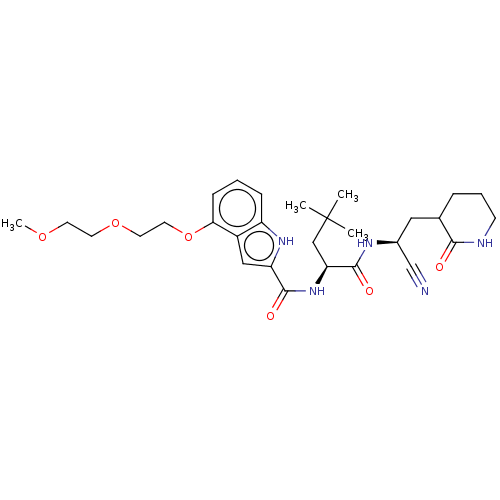 (US11524940, Compound 875 (Isomer 1)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds are assayed using standard methods to assess compound activity and IC50. As an exemplary for assessment of the SARS-COV2 Mpro, the C-His6-t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JD51M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 455 total ) | Next | Last >> |